
Thermodynamics studies the relationships between heat, work, and energy, governed by four fundamental laws. It describes energy conservation and the natural direction of energy flow.
Internal Energy (U)
Internal energy is the total energy contained within a system. It is the sum of the kinetic and potential energies of its particles. Importantly, we cannot measure the absolute value of internal energy; only changes in internal energy can be measured.State Function:
A state function depends only on the current state of the system, not the path taken to reach that state. Internal energy is a state function because its value at a particular state is independent of its history.Work (W) and Heat (Q)
Work and heat are the two primary means by which energy is transferred between a system and its surroundings. Work is energy transferred when a force is applied over a distance. Heat is energy transferred due to a temperature difference between a system and its surroundings.First Law of Thermodynamics:
The first law, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transferred or converted from one form to another. In terms of internal energy, heat, and work, it's expressed as:∆U = q – w
Where: ΔU is the change in internal energy. q is the heat added to the system. w is the work done by the system on its surroundings.Isothermal Reversible Process
An isothermal process occurs at a constant temperature. When it's reversible, the system remains in equilibrium with its surroundings throughout the process. For an ideal gas undergoing an isothermal reversible expansion or compression: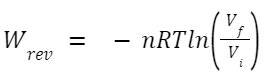 Where: n is the number of moles of gas.
R is the universal gas constant.
T is the constant temperature.
V
f
and V
i
are the final and initial volumes, respectively.
Where: n is the number of moles of gas.
R is the universal gas constant.
T is the constant temperature.
V
f
and V
i
are the final and initial volumes, respectively.
Download PDF Thermodynamics Formula
Isothermal Irreversible Process
In an isothermal irreversible process, the system doesn't necessarily remain in equilibrium with its surroundings. For an ideal gas undergoing an isothermal irreversible expansion against a constant external pressure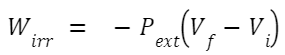
Free Expansion
Free expansion refers to the expansion of a gas into a vacuum, meaning there's no external pressure opposing the expansion. As a result, no work is done by or on the gas.W = 0
Also Check – Charle’s Law Formula
Adiabatic Process
An adiabatic process occurs without the transfer of heat. For an ideal gas undergoing an adiabatic expansion or compression, the work is described by: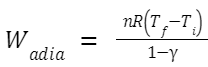 Where: γ is the heat capacity ratio
(Cp/Cv)
.
T
f
and T
i
are the final and initial temperatures, respectively.
Where: γ is the heat capacity ratio
(Cp/Cv)
.
T
f
and T
i
are the final and initial temperatures, respectively.
Enthalpy (H)
Enthalpy is a thermodynamic quantity defined as:H = U + PV
Where: U is the internal energy of the system. P is the pressure of the system. V is the volume of the system. For a process at constant pressure, the change in enthalpy (ΔH) is related to the heat (Q) transferred as:ΔH = Q P
Where Q P is the heat transferred at constant pressure.Also Check – S urface Chemistry Formula
Heat Capacity (C)
Heat capacity of a substance is the amount of heat (Q) required to raise its temperature by one degree Celsius (or Kelvin). Mathematically: C = Q/∆T Where ΔT is the change in temperature.Heat Capacity at Constant Pressure (C P ) and at Constant Volume (C V ): C P is the heat capacity measured at constant pressure. C V is the heat capacity measured at constant volume.
Relation with Ideal Gas Constant:
For an ideal gas, the molar heat capacities at constant pressure (C P ) and constant volume (C V ) are related to the ideal gas constant C P - C V = R Furthermore, the heat capacity ratio γ (often used in adiabatic processes for ideal gases) is defined as: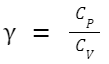
Also Check – Elevation of Boiling Point Formula
Entropy (S)
Entropy (S) is a measure of the disorder or randomness of a system. It provides insight into the energy of a system that is not available to do work. The concept of entropy is deeply rooted in the Second Law of Thermodynamics. Change in Entropy (ΔS) for Heat Transfer: For a reversible process in which heat is transferred: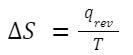 Where: q rev is the heat transferred during the reversible process. T is the absolute temperature at which the process occurs.
Where: q rev is the heat transferred during the reversible process. T is the absolute temperature at which the process occurs.
Entropy and Phase Changes: For a phase transition (e.g., melting or vaporization) at constant temperature:
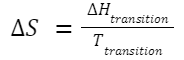
Spontaneity: The spontaneity of a process is determined by the Gibbs free energy
ΔG = ΔH − TΔS
Where: ΔG is the change in Gibbs free energy. ΔH is the change in enthalpy. ΔS is the change in entropy. T is the absolute temperature. The sign of ΔG dictates spontaneity: If ΔG<0 , the process is spontaneous. If ΔG>0 , the process is non-spontaneous. If ΔG=0 , the system is in equilibrium.Gibbs Free Energy (G)
Gibbs free energy is a thermodynamic potential that measures the maximum reversible work that can be performed by a system at constant temperature and pressure. It provides a criterion for the spontaneity of a process when both temperature and pressure are held constant. Formula forTemperature Dependence: The sign of ΔH and ΔS can give insights into the temperature dependence of spontaneity:
If ΔH<0 (exothermic) and ΔS>0 (entropy increases), the process is spontaneous at all temperatures. If ΔH>0 (endothermic) and ΔS<0 (entropy decreases), the process is non-spontaneous at all temperatures. If ΔH<0 and ΔS<0 , the process is spontaneous at low temperatures. If ΔH>0 and ΔS>0 , the process is spontaneous at high temperatures. The exact temperature at which the spontaneity changes can be found using the equation ΔG=0 , which givesT = ∆H/∆S
Thermodynamics Formula FAQs
Q1. What is entropy?
Q2. What is an adiabatic process?
Q3. How does the Second Law relate to entropy?
Q4. What is a reversible process?
Q5. Why can't the absolute value of internal energy be measured?










