
Ammonium dichromate formula , a bright orange crystalline solid, is highly flammable and leaves behind a green residue when burned. Heating it in a closed container can cause the container to break due to decomposition. It also acts as a powerful oxidizing agent when combined with combustible materials. When placed in water, it dissolves easily. Like other chromates and dichromates, it contains chromium in its hexavalent or +6 oxidation state. Now let's delve into the details of the Ammonium Dichromate Formula.
It is a salt that consists of both ammonium ions and dichromate ions at the same time. Due to its use in demonstrating tabletop 'volcanoes', ammonium dichromate is sometimes also referred to as 'vesuvian fire'. Due to its carcinogenic nature, however, this demonstration is not so popular in schools. During the early days of photography, some pyrotechnics used it as well.
Ammonium Dichromate Formula
Ammonium chromate has a different formula compared to ammonium dichromate. The formula for ammonium chromate is (NH4)2CrO4. In this compound, there is one ammonium cation and one chromate anion, [(CrO4)2-].
Also Read: Dinitrogen Trioxide Formula
Ammonium Dichromate Formula - Cation
The cation in the formula is ammonium, [(NH4)+]. Ammonium is a positively charged polyatomic ion consisting of one nitrogen atom and four hydrogen atoms. The overall charge of the ammonium cation is +1.
Also Read: Ethane Formula
Ammonium Chromate Formula - Ions
The ions in ammonium chromate are the ammonium cation, [(NH4)+], and the chromate anion, [(CrO4)2-]. These ions have distinct charges, with the ammonium cation carrying a +1 charge and the chromate anion having a -2 charge. When combined, they create a neutral compound, (NH4)2CrO4.
Ammonium Dichromate Formula by Criss Cross Method
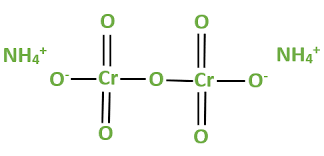
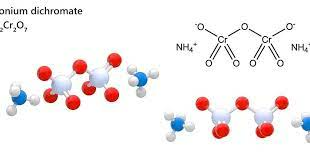
To derive the formula of ammonium dichromate using the criss-cross method, you need to balance the charges of the cations and anions. Since ammonium has a charge of +1 and dichromate has a charge of -2, you need two ammonium ions for each dichromate ion to achieve charge neutrality. This leads to the formula (NH4)2Cr2O7.
Ammonium Dichromate Formula Decomposes
Ammonium dichromate is known for its interesting property of spontaneous decomposition when heated. It decomposes into chromium(III) oxide, nitrogen gas, and water vapor. This decomposition is highly exothermic and often used for entertaining chemical demonstrations.
Ammonium Dichromate Formula - Atom
The formula (NH4)2Cr2O7 reveals the atomic composition of ammonium dichromate. It consists of atoms of nitrogen (N), hydrogen (H), and chromium (Cr), as well as oxygen (O) atoms in the dichromate anion.
Also Read: Sugar Formula
Ammonium Dic
hromate Formula Mass
The formula mass of ammonium chromate can be calculated by adding the atomic masses of all its constituent atoms. For (NH4)2CrO4, this involves the atomic masses of two nitrogen atoms (2 * 14.01 g/mol), eight hydrogen atoms (8 * 1.01 g/mol), one chromium atom (1 * 51.996 g/mol), and four oxygen atoms (4 * 15.999 g/mol).
Ammonium Dichromate Properties
Under standard conditions, the compound forms soluble orange acidic crystals. Its manufacturing method involves using chromic acid to react with ammonium hydroxide, followed by crystallization. The crystal structure of (NH4)2Cr2O7 (C2/c, z=4) contains a single type of ammonium ion. Each NH4+ center is surrounded by 8 oxygen atoms at N—O distances ranging from approximately 2.83 – 3.17 Å, which are typical for hydrogen bonds at symmetry site C1 (2,3).Ammonium Dichromate Uses
Ammonium dichromate has many uses, such as in pyrotechnics and historically in photography and lithography. It is also utilized as a source of pure nitrogen in chemistry labs, a catalyst, and a mordant in dyeing processes for pigments like alizarin and chrome alum. In industries, it plays a role in leather tanning and oil purification. Additionally, it is incorporated into photosensitive films containing PVA and phosphor for TV screens, LCDs, and other devices. The ammonium dichromate serves as the photoactive site in these cases.| Related Links | |
| Sodium Citrate formula | Carbonous Acid Formula |
| Sodium Acetate formula | Chlorine Gas Formula |
Ammonium Dichromate Formula FAQs
What is the formula of ammonium dichromate?
What are the atoms in ammonium dichromate?
How many atoms are in (NH4)2Cr2O7?
What is the state of ammonium dichromate?










