
The water formula is "H2O." This name is derived from the chemical symbols of its constituent elements: hydrogen (H) and oxygen (O). The number "2" in the water formula indicates that there are two hydrogen atoms for every one oxygen atom in a water molecule.
Water Formula H2O
The chemical formula H2O is often called the molecular formula of water. It signifies that a water molecule comprises two hydrogen atoms (H) and one oxygen atom (O) bonded together. The molecular formula is a concise way to represent the elemental composition of water.
Also Read : Glycerol Formula
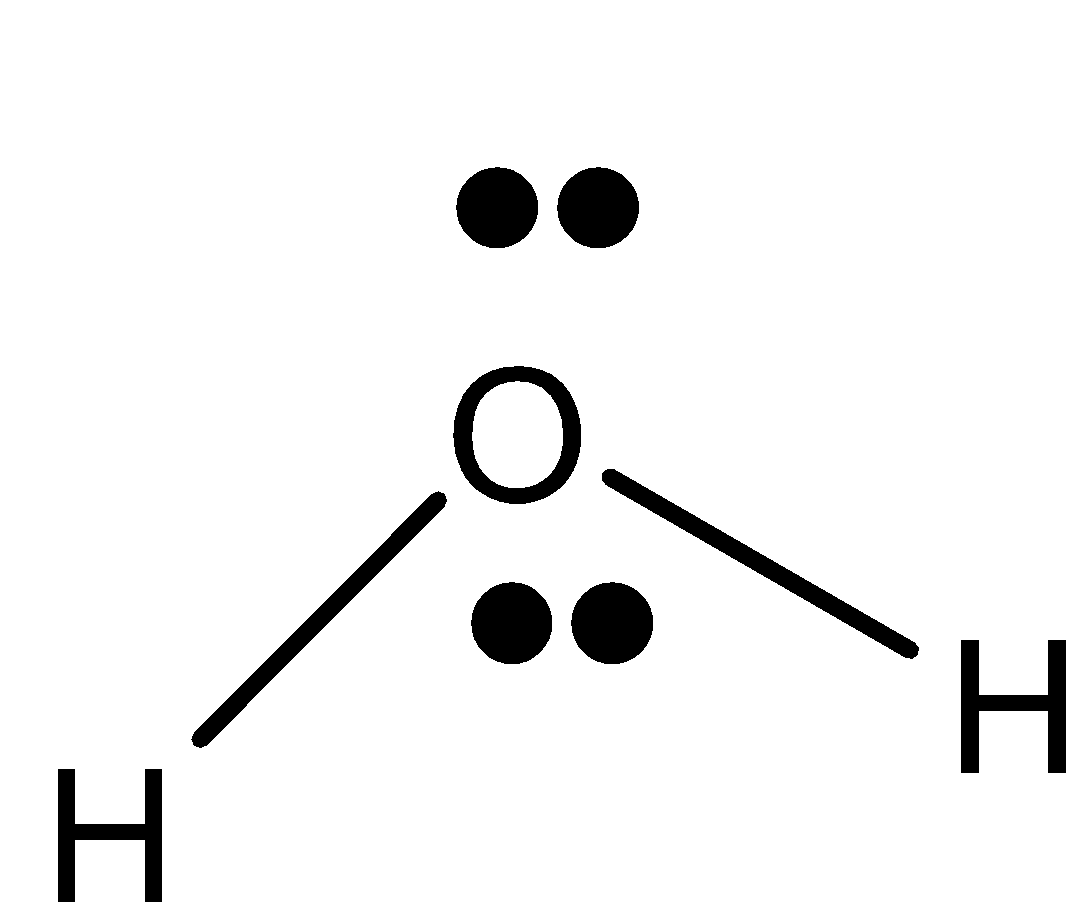
Structure of water
The water formula is composed of two hydrogen atoms, each linked by a single chemical bond to an oxygen atom. Most hydrogen atoms have a nucleus consisting solely of a proton. Water contains two isotopic forms, tritium and deuterium, which have one neutron in their nuclei and one neutron in their nucleus. Some nuclear reactors use deuterium oxide (D2O) as a neutron moderator to moderate neutrons. It is important in chemical research and is often used as a neutron moderator. In spite of its simple formula (H2O), water formula has a very complex chemical and physical structure. Comparing it with analogous compounds, such as hydrogen sulfide and ammonia, the melting point and boiling point are much higher than expected. Another unusual property of water when it is solid, ice, is that it is less dense than when it is liquid. The electronic structure of the water molecule explains these anomalies. There is a special bend in the water molecule. The two hydrogen atoms are bound to the oxygen atom at a 104.5° angle. The hydrogen atoms are bound at an angle of 104.5 degrees to the oxygen atom in the water molecule.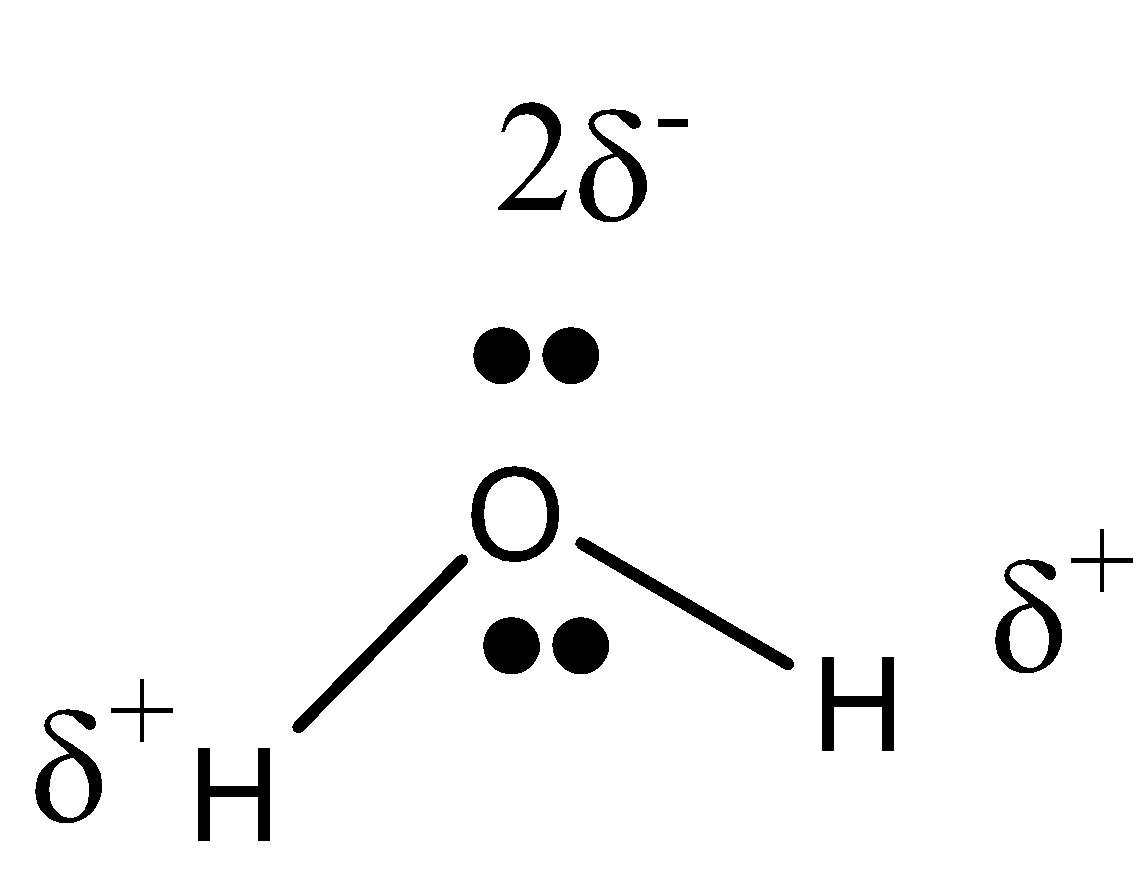
Also Read : Hexane Formula
Water Formula in Science
Water is vital in various scientific disciplines, including chemistry, biology, and environmental science. Its formula, H2O, is central to understanding the properties and behaviours of water in different scientific contexts. Water's unique molecular structure gives rise to its exceptional properties, such as high surface tension, thermal capacity, and solvent capabilities.
Water Formula Equation
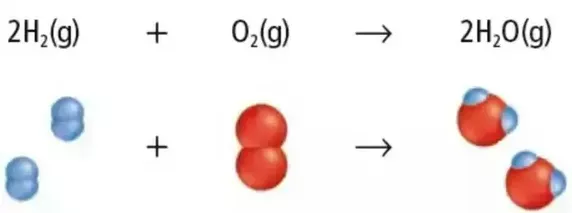
The chemical equation for the formation of water can be represented as follows:
2H2 + O2 → 2H2O
This equation illustrates the synthesis of water from its constituent elements, hydrogen (H) and oxygen (O). It shows that two molecules of hydrogen gas (H2) react with one molecule of oxygen gas (O2) to produce two molecules of water (H2O).
Water Formula Reaction
Water formation, often called a combustion or oxidation reaction, is a fundamental chemical process. It involves breaking the diatomic bonds in hydrogen and oxygen gases and the formation of new bonds in water molecules. This reaction releases energy and is critical to various industrial processes and combustion engines.
Water Formula Name in Chemistry
Water is commonly known as dihydrogen monoxide in chemistry, reflecting its molecular composition. This name emphasizes the presence of two hydrogen atoms (dihydrogen) and one oxygen atom (monoxide) in each water molecule. While this name is rarely used, it systematically describes water's composition based on its chemical formula, H2O.
Also Read : Hydrocyanic Acid Formula
Physical Properties of Water
Water possesses various significant physical characteristics, which may be well-known due to its universal presence. However, the majority of these properties are not typical. Despite having a relatively low molar mass, water displays exceptionally high values for viscosity, surface tension, heat of vaporization, and entropy of vaporization, all of which can be attributed to the extensive hydrogen bonding in liquid water. The unique arrangement of hydrogen bonds in solid ice is responsible for its lower density than liquid water, a rare occurrence among commonly found substances.
| Physical properties of water | |
|---|---|
| molar mass of water | 18.0151 grams per mole |
| melting point of water | 0.00 °C |
| boiling point of water | 100.00 °C |
| maximum density (at 3.98 °C) | 1.0000 grams per cubic centimetre |
| density (25 °C) | 0.99701 grams per cubic centimetre |
| vapour pressure (25 °C) | 23.75 torr |
| heat of fusion (0 °C) | 6.010 kilojoules per mole |
| heat of vaporization (100 °C) | 40.65 kilojoules per mole |
| heat of formation (25 °C) | −285.85 kilojoules per mole |
| entropy of vaporization (25 °C) | 118.8 joules per °C mole |
| viscosity of water | 0.8903 centipoise |
| surface tension (25 °C) | 71.97 dynes per centimeter |
| Related Links | |
| Butan 1 ol Formula | Carbon Chemical Formula |
| Butyric Acid Formula | Sodium Citrate formula |
Water Formula FAQs
What is H2O full form?
How is H2O water?
What is H2O structure?
What is 2H2O called?










