
The ammonium iodide formula is NH4I and has multiple uses in photographic chemicals and medications. Ammonium Iodide Formula preparation involves combining hydroiodic acid with ammonia. It readily dissolves and forms cubic crystals when mixed with water (H2O). Additionally, it can be dissolved in ethanol solution and will turn yellow upon exposure to moist air due to decomposition and release of iodine.
Ammonium Iodide Formula
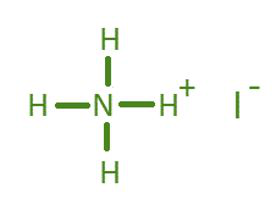
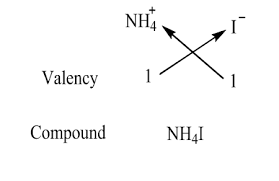
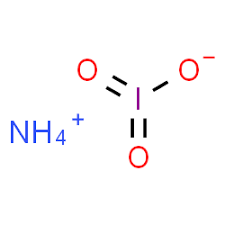
Ammonium iodide is a chemical compound with the formula NH4I. This formula represents the composition of the compound, showing that it consists of one ammonium cation [(NH4)+] and one iodide anion [(I)-].
Also Read: Cyanide Formula
Ammonium Iodide Formula - Ionic Compound
Ammonium iodide is an ionic compound. It is formed through the combination of an ammonium cation (a positively charged ion) and an iodide anion (a negatively charged ion). The electrostatic attraction between these oppositely charged ions holds the compound together.
Also Read: Butyric Acid Formula
Ammonium Iodide Formula by the Crisscross Method
The crisscross method can be used to determine the formula of ammonium iodide. Ammonium has a charge of +1, and iodide has a charge of -1. You simply need one ammonium cation for one iodide anion to balance the charges, resulting in the formula NH4I.
Ammonium Iodate Formula
Ammonium iodide should not be confused with ammonium iodate, which has a different chemical formula. The formula for ammonium iodate is (NH4)2IO3, indicating that it contains two ammonium cations and one iodate anion.
Ammonium Iodide Formula - Ionic or Covalent
Ammonium iodide is an ionic compound, as it is composed of ions with opposite charges. The ammonium ion [(NH4)+] is formed through a covalent bond within the ion, but when combined with the iodide anion [(I)-], it forms an ionic bond.
Also Read :Ammonium Iodide Chemical Formula
The chemical formula of ammonium iodide is NH4I. This compound is commonly used in various chemical applications, including as a source of iodine in chemical reactions or for photographic purposes. It is a white crystalline salt that is soluble in water.
Also Read: Carbonous Acid Formula
Preparation of Ammonium Iodide
Our first step is to dissolve 254 grams of potassium iodide in 125 milliliters of water (H2O) and mix it with 100 milliliters of ammonium sulfate. Then we have to dissolve it in boiling water. Once it has cooled down, we add 40 milliliters of alcohol to it and keep it for 12 hours.
As a result, we obtain a precipitate of potassium sulfate that we filter, then we evaporate the filtrate occasionally by adding ammonia water with a small amount of alcohol until crystallization occurs. In addition, we wash the potassium sulfate crystals with dilute alcohol, which contains about 20% alcohol. Furthermore, after combining the filtrates and evaporating, we obtain ammonium iodide.
The equation is (NH4)2SO4 + 2KI →2NH4I + K2SO4
As ammonium iodide crystallizes into cubes and deliquesces in the air, it must be preserved in a dark place because the light decomposes the salt, causing it to turn brown.
To crystallize, the solution is treated with ammonium sulfide, filtered, and evaporated with a steam bath. The aqueous solution is readily soluble in alcohol. As a result of the decomposition and separation of the iodine, the solution turns yellow when standing for some time. One part of the salt dissolves in 0.60 parts of water (H2O).
Ammonium iodide can also be made in a laboratory by reacting ammonia or ammonium hydroxide with hydroiodic acid or hydrogen iodide:
A combination of NH3+HI → NH4I
It is calculated by multiplying NH4OH + HI → NH4I + H2O.
Also Read: Cholesterol Formula
Properties of Ammonium Iodide
Besides being visible as a white crystalline powder, ammonium iodide has a melting point of 551 °C and a boiling point of 405 °C, a molar mass of 144.94 g/mol, a density of 2.51 g/cm3, and is easily soluble when put into water (H2O).
| Properties of Ammonium Iodide | |
| Name | Ammonium Iodide |
| Appearance | White crystalline powder |
| Chemical Formula | NH 4 I |
| Melting Point | 551 °C |
| Boiling Point | 235 °C |
| Molar Mass | 144.94 g/mol |
| Density | 2.51 g/cm³ |
| Solubility in Water | Soluble |
| Related Links | |
| Copper II Carbonate Formula | Resorcinol Acid formula |
| Silver Acetate formula | Propan-2-Ol formula |
Ammonium Iodide Formula FAQs
How do you write ammonium iodide?
What is the chemical name for ammonium iodide?
Is ammonium iodide a salt?
What is the formation of ammonium iodide?










