Difference Between Oxidation and Reduction: Learning about oxidation and reduction is super important in chemistry. These concepts are like the building blocks for redox reactions, which you can see everywhere in the chemical processes.
| NEET Chemistry Syllabus | NEET Chemistry Important Questions with Answers |
| NEET Chemistry Chapter wise Weightage | NEET Chemistry MCQs |
| NEET Chemistry Notes | NEET Chemistry Formulas |
Difference Between Oxidation and Reduction Overview
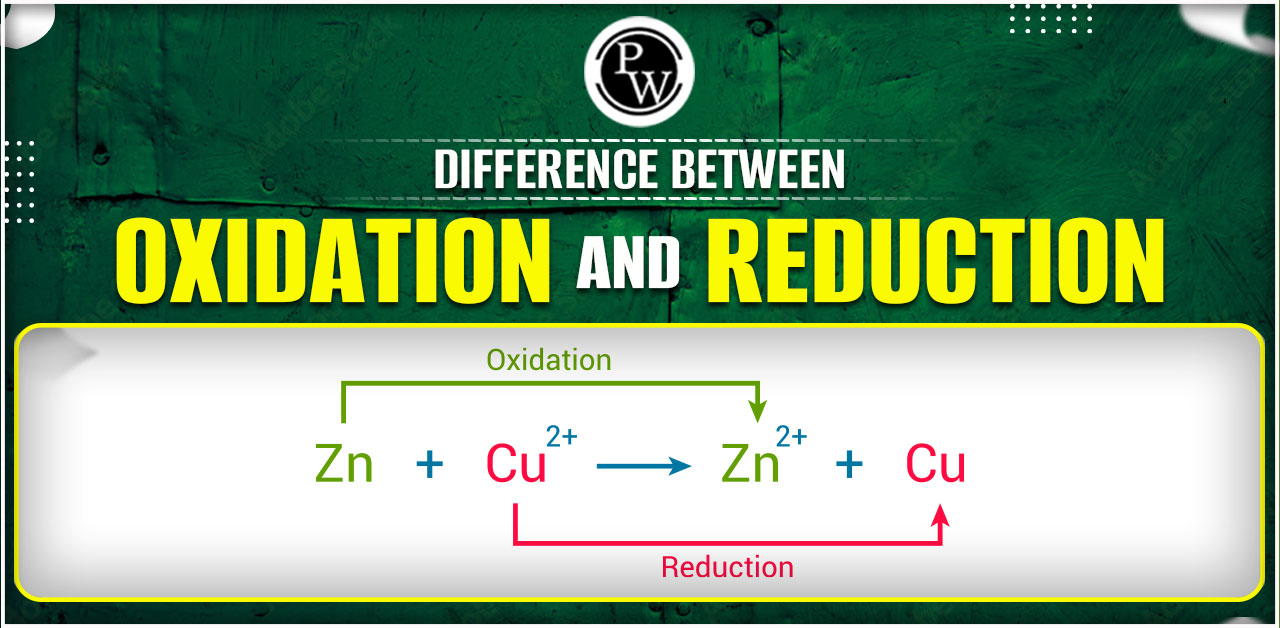
Oxidation and reduction are like two sides of a coin. Oxidation happens when something loses electrons or gains oxygen, and reduction occurs when something gains electrons or loses oxygen. They often go hand in hand in chemical reactions, creating a balanced exchange of electrons. These processes, called redox reactions, are important in everyday scenarios like rust forming or how our bodies produce energy. Knowing the basics of oxidation and reduction helps us understand how substances change and interact in the world around us.Difference Between Oxidation and Reduction
Below is a table illustrating the distinctions between oxidation and reduction, highlighting the basics of these fundamental chemical processes. NEET aspirants can understand the process of oxidation and reduction and the real difference between them.| Difference Between Oxidation and Reduction | ||
|---|---|---|
| Parameters | Oxidation | Reduction |
| Definition | Loss of electrons or increase in oxidation state. | Gain of electrons or decrease in oxidation state. |
| Electron Transfer | Electrons are released or donated. | Electrons are gained or accepted. |
| Involvement of Oxygen | Can involve the addition of oxygen to a substance. | May involve the removal of oxygen from a substance. |
| Involvement of Hydrogen | Can involve the removal of hydrogen from a substance. | May involve the addition of hydrogen to a substance. |
| Change in Oxidation State | Increase in oxidation state. | Decrease in oxidation state. |
| Reducing Agent | Substance getting oxidized. | Substance getting reduced. |
| Oxidizing Agent | Substance getting reduced. | Substance getting oxidized. |
| Common Example | Rusting of iron: Fe → Fe²⁺ + 2e⁻ (oxidation) | Reduction of copper(II) ions: Cu²⁺ + 2e⁻ → Cu (reduction) |
What is Oxidation?
Oxidation refers to the loss of electrons or an increase in oxidation state during a chemical reaction. One real-life example is the process of rusting. When iron (Fe) reacts with oxygen (O2) in the presence of water (H2O), it undergoes oxidation, producing iron oxide (rust) and releasing electrons.What is Reduction?
Reduction involves the gain of electrons or a decrease in oxidation state during a chemical reaction. This reduction process occurs during electroplating when a metal object, such as a key or jewelry, is immersed in a copper sulfate solution. The application of an electric current facilitates the reduction of copper ions onto the surface of the object, resulting in the deposition of a thin layer of copper. In a chemical reaction, pay attention to how much an element shares its electrons. If it shares less, it's getting oxidized (like losing a bit of weight). If it shares more, it's getting reduced. So, if an element's "sharing electron" goes up, it's losing, and if it goes down, it's gaining.Difference Between Oxidation and Reduction FAQs
What is oxidation and reduction in simple terms?
Oxidation involves losing electrons or gaining oxygen, while reduction involves gaining electrons or losing oxygen. It's like a chemical exchange of electrons between substances.
How do oxidation and reduction work together in a reaction?
Oxidation and reduction always happen together in what's called a redox reaction. One substance loses electrons (oxidation), and another gains them (reduction)
Can you give a real-life example of oxidation and reduction?
Sure, rusting (iron turning into rust) is an example of oxidation, and electroplating (coating a metal object with another metal) is an example of reduction.
Why are oxidation and reduction important in chemistry?
They are fundamental to understanding how substances change and react. Redox reactions are widespread in chemistry, from producing energy in our bodies to various industrial processes.
How can oxidation and reduction be identified in a chemical equation?
Look for changes in oxidation states. If an element's oxidation state increases, it's oxidized; if it decreases, it's reduced. This helps spot oxidation and reduction in a reaction.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.