
The chemical formula for Ammonium Carbonate , also known as Baker's Ammonia Formula or diammonium salt formula, is (NH4)2CO3. It is a chemical compound that is composed of ammonium and carbonate ions.
Colorless crystal or white powder, it has a strong odour of ammonia and a sharp ammoniacal taste. It is non-combustible and soluble in water. When it reacts with acids, it forms ammonium salts and carbon dioxide. When it reacts with bases, it produces ammonia gas. Carbon dioxide and aqueous ammonia are blended to produce it.
Due to its property of degrading to gaseous ammonia and carbon dioxide when heated, it is widely used as a leavening agent and smelling salt.
Ammonium Carbonate Formula
(NH 4 ) 2 CO 3 is the chemical formula of ammonium carbonate, a white, crystalline salt.
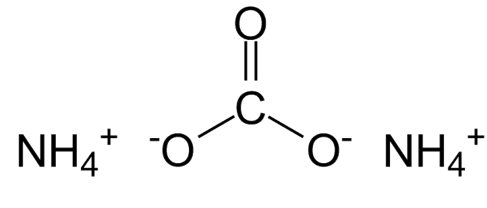
Molecular Formula of Ammonium Carbonate
Ammonium Carbonate has a chemical formula or molecular formula of (NH4)2CO3. It degrades into gaseous ammonia and carbon dioxide when heated at high temperatures. It is primarily used as a leavening salt and aroma salt. It was the precursor to modern leavening agents like baking soda and baking powder.
Production
To produce ammonium carbonate, carbon dioxide and aqueous ammonia are combined. This process yields approximately 80000 tons per year, as of 1997. The gas required for creating ammonium carbonate must be high in purity, ranging from 99% to 99.99%. It is then sent into two buffer gas vats with a pressure between 0.2 and 2MPa. From there, the gas is released through the Rota meter valve and into a sealed PVC synthesis reaction bag where it forms the final product. The flow rate for this process is (0.6-2) X 106L/h overall, with (0.1-0.6) X 106L/h for carbon dioxide and ammonia separately.
Also Check – Potassium Cyanide formula
Decomposition
With standard temperature and pressure, ammonium carbonate decomposes very slowly through two pathways. In the end, a sample of ammonium carbonate that is initially pure will soon turn into a mixture that contains a number of by-products. In addition to ammonium bicarbonate and ammonia, ammonium carbonate can spontaneously decompose.
(NH4)2CO3 → NH4HCO3 + NH3
Later on, they decompose into carbon dioxide, water, and other ammonia molecules.
NH4HCO3 → H2O + CO2 + NH3
Also Check – Malic Acid formula
Properties Of Ammonium Carbonate (Baker’s Ammonia)
| The chemical formula of Ammonium Carbonate | (NH 4 ) 2 CO 3 |
| Molecular weight | 96.09 g/mol |
| Density | 1.50 g/cm3 |
| Melting point | 58 °C |
| Boiling point | Decomposes |
Also Read – Cobalt II Nitrate Formula
Uses
Leavening Agent
In some traditional recipes, especially those from northern Europe and Scandinavia, such as Speculoos, Tunnbröd and Lebkuchen, Ammonium carbonate also functions as a leavening agent. It is the ancestor of general baking powder which is used today.
Some Other Uses
Ammonium carbonate, the primary element of smelling salts, is also used in other products. Buckley's cough syrup, produced in Canada, uses this ingredient to alleviate bronchitis symptoms. It has emetic properties and can also be found in smokeless tobacco and as a lens cleaner for cameras such as Eastman Kodak's "Kodak Lens Cleaner."
Ammonium Carbonate Formula FAQs
Q1. What is the chemical formula for Ammonium Carbonate?
Q2. Is Ammonium Carbonate a stable compound?
Q3. What are some common uses of Ammonium Carbonate?
Q4. Is Ammonium Carbonate soluble in water?
Q5. Why is Ammonium Carbonate used in some traditional baking recipes?










