

Chromates are the salts of chromic acid that contain the chromate anion with the chemical formula CrO42– and usually have an intense yellow color. Sodium and potassium salts of chromate are the most commonly used chromate salts. The oxoanion is generated when protons are removed from chromic acid. It is also known as chromium oxoanion and divalent inorganic anions.
Chromate Structure – CrO 42-
As a result of the removal of two protons from chromic acid, chromate is the salt of chromic acids. The chromium oxo anion is converted into a divalent inorganic anion with the chemical formula CrO42-. Many polyatomic ions are used to form insoluble salts. Among the chromate salts are sodium salts and potassium salts. Chromate has an intense yellow color and is a powerful oxidizing agent.Chromate Ion
In the realm of chemistry, chromate falls in the category of inorganic salts. Acids and bases combine to create neutral ionic compounds called salts. Those lacking carbon-hydrogen bonds are referred to as inorganic salts, while those containing these bonds are known as organic salts. When an acid interacts with a base, it produces inorganic salts. Additionally, acids may also react with metals, forming inorganic salts. Some well-known examples of mineral salts include sodium chloride, potassium chloride, calcium chloride, and others formed by combining sodium, potassium, or calcium with chlorine.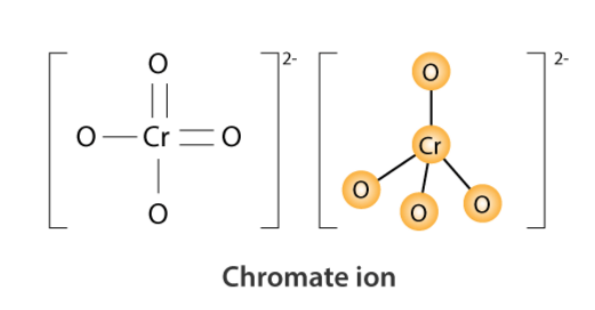 The acid A and the base B combine to form salt AB and release water. AH+BOH = AB+H2O
The acid A and the base B combine to form salt AB and release water. AH+BOH = AB+H2O
Also Check - Chloroacetic Acid Formula
What is Chromate Formula?
Chromate, also known as chromium oxoanions, is an inorganic salt containing chromic acid with a distinctive yellow hue. Its oxidation state is six plus, and it is formed when protons are removed from chromic acid. The IUPAC name for chromate is dioxo chromium, and its molecular weight is 194.18 g/mol. The formula for chromate anion is CrO42-.| CrO42- | Chromate |
| Density | 2.73 g/cm³ |
| Molecular Weight/ Molar Mass | 194.1896 g/mol |
| Conjugate acid | Chromic acid (H2CrO4) |
| Oxidation State | +6 |
| Chemical Formula | CrO42- |
Also Check - Urethane Formula
Physical Properties of Chromate – CrO42-
| Odour | Odourless |
| Appearance | Yellow powder |
| Valency | 2 |
| Solubility | Generally insoluble in water. |
Chemical Properties of Chromate
Chromate has a variety of chemical properties. It also has a variation known as dichromate salts that possess dichromate anion. In acidic solutions, its ion works as a firm oxidizing agent. Upon combining it with water, we can observe that it forms chromium(III) hydroxide. CrO42- + 4H2O + 3e- ---> Cr(OH)3 + 5OH- We can create potassium nitrate and barium chromate by reacting potassium chromate with barium nitrate. K2CrO4 + Ba(NO3)2 <---> BaCrO4+ 2KNO3 It also combines with other elements to form ammonium, sodium, and calcium chromate.Also Check - Acetylene Formula
Uses of Chromate Formula
Chromate has many uses, including forming a pretreatment layer to create a protective coating on surfaces. These salts, such as potassium, sodium, calcium, and ammonium, have excellent corrosive properties and serve as an adhesive between metal and primer to prevent corrosion. Despite their long-lasting effects and self-healing capabilities, they can deplete upon damage. Industries commonly deposit these coatings on materials, although it is important to note that chromate can be hazardous to health. However, this method is still used in products such as rustproofing materials and enamels for corrosion prevention. The salts can also form pigments for ink or dye due to their oxidizing property and low solubility. As they are highly toxic for health, it is important not to consume these products. In fact, even industrial crayons may contain chromate salts that are harmful if ingested. Another common use of chromate is in the process of chromate plating, which protects everyday items from rust and decay by utilizing the beneficial properties of chromium salts.Chromate Formula FAQs
What is the structural formula of chromate ion (CrO4^2-)?
The structural formula of the chromate ion (CrO4^2-) consists of one chromium (Cr) atom bonded to four oxygen (O) atoms in a tetrahedral arrangement.
What is the charge on the chromate ion (CrO4^2-)?
The chromate ion carries a charge of -2, indicated by the superscript 2- in its chemical formula. This charge results from combining one chromium atom with four oxygen atoms.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.
Get App











