The hyponitrous acid formula is H2N2O2, is an intriguing and relatively rare chemical compound. This weak acid belongs to a family of nitrogen oxoacids and plays a significant role in various chemical reactions and processes. In this article, we will explore the Hyponitrous acid formula, structure, properties, preparation, uses, and potential hazards associated with hyponitrous acid, shedding light on its importance in the world of chemistry.
Hyponitrous Acid Formula
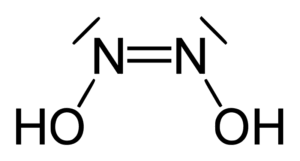
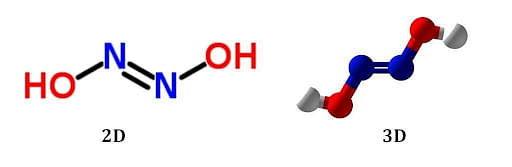
The chemical formula for hyponitrous acid is H2N2O2. This formula reflects the presence of two hydrogen (H) atoms, two nitrogen (N) atoms, and two oxygen (O) atoms in each molecule of hyponitrous acid. The presence of multiple nitrogen and oxygen atoms in its formula contributes to its unique properties and reactivity.
Also Read: Pentane Formula
Hyponitrous Acid Formula Structure
The arrangement of its constituent atoms characterizes the structure of hyponitrous acid. It consists of two nitrogen atoms (N) connected by a single covalent bond (N-N) and flanked by two oxygen atoms (O) bonded to the nitrogen atoms. The two hydrogen atoms (H) are attached to the oxygen atoms. This results in a simple linear structure for the hyponitrous acid molecule. The presence of the N-N single bond distinguishes it from other nitrogen oxoacids, such as nitrous acid and nitric acid.
Also Read: Oxalic Acid Formula
Properties of Hyponitrous Acid Formula
Hyponitrous acid is a weak and unstable acid. It is a colorless or pale yellow liquid with a faint odor. It is sparingly soluble in water and tends to decompose, especially at higher temperatures, into various nitrogen oxides and water. Due to its instability, it is challenging to isolate and store in a pure form. Hyponitrous acid is a diprotic acid that can donate two protons (H⁺ ions) in aqueous solutions.
| Properties of Hyponitrous Acid Formula | |
| Appearance | Liquid |
| odour | Pungent |
| Hyponitrous acid Formula | H2N2O2 |
| IUPAC name name for Hyponitrous acid Formula | Diazenediol |
| Molecular weight | 62.0282 g/mol |
| Conjugate base | Hyponitrite |
| H bond acceptors | 4 |
| H bond donors | 2 |
Preparation of Hyponitrous Acid
Hyponitrous acid can be prepared through various methods, often involving the reaction of sodium nitrite (NaNO2) with an acid, such as hydrochloric acid (HCl). The reaction typically yields hyponitrous acid as an intermediate product, which can further react or decompose depending on the reaction conditions.
In the presence of ether medium, silver (I) hyponitrite (Ag2N2O2) and anhydrous hydrochloric acid (HCl) can be used to synthesize hyponitrous acid.Ag 2 N 2 O 2 +2HCl→ H 2 N 2 O 2 +2AgCl
During this reaction, ether evaporates, resulting in solid H2N2O2, but the acid (silver chloride) remains unstable, resulting in an explosion chain that continues. As a result of the following reaction, the acid decomposes in an aqueous solution to form anhydride of hyponitrous acid:H 2 N 2 O 2 → H 2 O+ N 2 O
Because N2O is anhydrite, aqueous solutions (water) cannot be used to prepare the acid in the above reaction. Therefore, hyponitrous acid is treated with air to obtain nitric and nitrous acid:2 H 2 N 2 O 2 + 3 O 2 → 2 HNO 3 + 2 HNO 2
Hyponitrous acid is formed by reducing nitrate or nitrite in a water medium with sodium amalgam as a catalyst, leading to the formation of Cis and Trans structures.Also Read: Methane Formula
Hyponitrous Acid Uses
Hyponitrous acid is primarily used as an intermediate in chemical synthesis. It serves as a precursor in the production of various compounds, including pharmaceuticals and dyes. However, its applications are limited compared to some other nitrogen oxoacids due to its instability and the challenges associated with handling and storing it.
Also Read: Propionic Acid Formula
Hazards of Hyponitrous Acid
Hyponitrous acid is not commonly encountered in its pure form due to its reactivity and instability. However, it is important to note that its decomposition products, which include nitrogen oxides, can be toxic and harmful to human health and the environment. Proper precautions and safety measures must be taken when working with compounds that may generate hyponitrous acid as an intermediate. Additionally, its instability makes it challenging to handle safely.
| Related Links | |
| Cinnamic Acid Formula | Carbon Monoxide Formula |
| Trichloroacetic Acid formula | Chloroacetic Acid Formula |
Hyponitrous Acid Formula FAQs
What is the formula for hydronitrous acid?
What is Hydronitrous acid?
What is the chemical formula of Hyposulfurous acid?
How is hyponitrous acid prepared?