
The lactic acid formula is C₃H₆O₃ , it is a simple organic acid, that encapsulates its basic molecular composition. Lactic acid formula, a key component in various biological processes, is crucial in chemistry, biology, and food production. This versatile compound has a unique structure and is produced through a fascinating process known as lactic acid fermentation.
Lactic Acid formula can be dissected into its constituent elements: C₃: This signifies that lactic acid consists of three carbon (C) atoms, forming the backbone of its molecular structure. H₆: This denotes the presence of six hydrogen (H) atoms bonded to the carbon atoms. These hydrogen atoms contribute to the compound's reactivity. O₃: The formula also includes three oxygen (O) atoms, which are incorporated into the compound's functional groups. Lactic acid, in its true form, exists as both L-lactic acid and D-lactic acid, which are mirror-image isomers. It can also appear as a racemic mixture, where both forms coexist in equal proportions. Its molecular structure includes a carboxyl group (COOH) at one end and a hydroxyl group (OH) on the adjacent carbon atom. The arrangement of these functional groups contributes to lactic acid's properties and reactivity, making it a versatile compound with diverse applications in various fields, including food production, skincare, pharmaceuticals, and more.Lactic Acid Formula
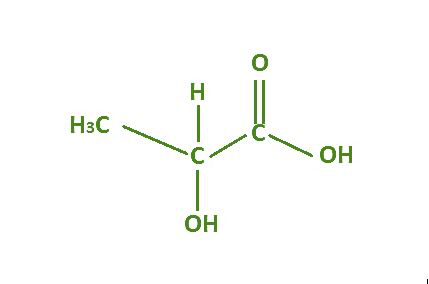
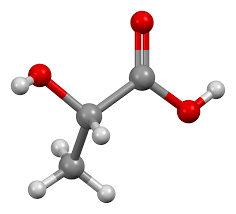
Lactic acid is a simple organic acid with the chemical formula C₃H₆O₃. It is classified as a carboxylic acid due to the presence of a carboxyl group (COOH) in its molecular structure. Lactic acid formula reflects the basic composition of lactic acid, consisting of three carbon atoms (C), six hydrogen atoms (H), and three oxygen atoms (O).
Also Read: Dimethylglyoxime Formula
Lactic Acid Formula Structure
Lactic acid formula structure is intriguing and important in understanding its properties and reactivity. Lactic acid exists in two enantiomeric forms, L-lactic acid (levorotatory) and D-lactic acid (dextrorotatory), which are mirror images of each other. These isomers can also exist as a racemic mixture, where equal amounts of both forms are present. Lactic acid molecules form a chain, with the carboxyl group at one end and a hydroxyl group (OH) on the adjacent carbon atom. This structure is responsible for its acidity and reactivity.
Also Read: Potassium Acetate formula
Lactic Acid Fermentation
Lactic acid fermentation is a biological process that converts glucose and other carbohydrates into lactic acid. It occurs in various microorganisms, including certain types of bacteria and fungi. This fermentation process plays a significant role in the production of various food and beverage items, such as yogurt, cheese, sauerkraut, and pickles. It is also a key metabolic pathway in the human body, particularly during intense exercise when oxygen availability is limited. Lactic acid produced during anaerobic respiration is responsible for muscle fatigue and soreness.
Lactic Acid Bacteria
Lactic acid bacteria (LAB) are a group of microorganisms that play a pivotal role in lactic acid fermentation. These bacteria are found in various environments, including the human digestive system, plant surfaces, and food products. LAB have the unique ability to convert sugars into lactic acid, contributing to the preservation and flavor development in fermented foods. Some well-known LAB species include Lactobacillus, Streptococcus, and Bifidobacterium.
Also Read: Oxalic Acid Formula
Lactic Acid Formula Uses
Lactic acid formula finds application in a wide range of industries thanks to its unique properties and characteristics. Some of its key uses include:
- Food and Beverage Production:
- Lactic acid is used as a food preservative and a flavor enhancer in various fermented products.
- It is a key component in the production of dairy items like yogurt and cheese.
- Cosmetics and Personal Care:
- Lactic acid is a common ingredient in skin care products, such as exfoliating agents and moisturizers, owing to its mild exfoliating and hydrating properties.
- Pharmaceutical and Medical:
- In the pharmaceutical industry, lactic acid is used as an excipient in formulating various medications.
- It is also employed to prepare biodegradable polymers for drug delivery systems.
- Textile and Leather:
- Lactic acid-based polymers are used in the textile and leather industries for various applications, including fabric finishing and tanning.
| Related Links | |
| Ascorbic Acid formula | Acetaldehyde Formula |
| Butane Formula | Acetonitrile formula |
Lactic Acid Formula FAQs
What is the full formula of lactic acid?
What is the formula for lactic acid for the skin?
Why is lactic acid called milk acid?
What is the pH value of lactic acid?










