
Ammonium Phosphate Formula: The formula for Ammonium Phosphate is (NH 4 ) 3 PO 4 . which is formed by combining ammonia and phosphoric acid. This compound is a colorless, unstable salt with a crystalline structure, and it easily dissolves in water.
The Ammonium ion carries a positive charge of 1+.while the Phosphate ion carries a charge of 3–. Upon charge balancing, the resulting formula is (NH 4 ) 3 PO 4 . This article will cover the structure, properties, applications, and methods of preparing this compound.
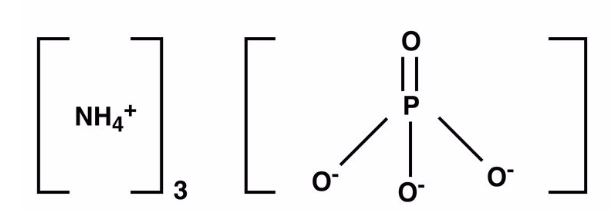
Ammonium Phosphate Formula Structure
The Ammonium Phosphate structure is described by the chemical formula (NH 4 ) 3 PO 4 , Its molar mass is 149.09 g/mol. This compound contains ammonium cations (NH4+). which are positively charged, and phosphate anions (PO 4 3- ), which carry a -3 formal charge. By combining these ions in a three-to-one ratio or cross-multiplying their ionic charges, we arrive at this formula. Below are structure of ammonium phosphate.
Ammonium Phosphate Formula Physical Properties
Below are physical properties of ammonium phosphate.
Ammonium Phosphate is represented by the molecular formula (NH 4 ) 3 PO 4 .
Ammonium Phosphate is a white crystalline solid with a white appearance.
Its density is 1.619 g/cm3 and Its molar mass is 149.09 g/mol.
It is easily soluble in water and exhibits a pH range of 4-4.5.
Its melting point is 155°C
Its boiling point is 130°C.
Ammonium Phosphate Formula Chemical Properties
Ammonium Phosphate Formula is (NH 4 ) 3 PO 4 . Ammonium Phosphate exhibits distinct chemical properties. When it goes to decomposition. It produces phosphoric acid and ammonia, accompanied by the release of toxic gases. The chemical reaction can be represented as follows:
(NH 4 ) 3 PO 4 → 3NH 3 + H 3 PO 4
In its reaction with zinc nitrate. Ammonium Phosphate produces zinc phosphate and ammonium nitrate as products. This reaction can be expressed as:
Zn(NO 3 ) 2 + 2(NH 4 )PO 4 → Zn 3 (PO 4 ) 2 + 6NH 4 NO 3
Moreover, when Ammonium Phosphate reacts with lead nitrate. It triggers a double displacement reaction, leading to the formation of lead phosphate and ammonium nitrate as the resulting products.
3Pb(NO 3 ) 2 + 2(NH 4 ) 3 PO 4 → Pb(PO 4 ) 2 + 6NH 4 NO 3
Preparation of Ammonium Phosphate
Ammonium Phosphate Formula is (NH 4 ) 3 PO 4 . Ammonium Phosphate is prepared by treating a solution of phosphoric acid and ammonia. The reaction can be described as follows:
3NH 3 + H 3 PO 4 → (NH 4 ) 3 PO 4
This involves a neutralization reaction, where ammonia and phosphoric acid combine to yield monoammonium phosphate as the initial product. Subsequently, this compound is further reacted with ammonia to produce diammonium phosphate, and the chemical reactions can be summarized as:
NH 3 + H 3 PO 4 → NH 4 H 2 PO 4
NH 4 H 2 PO 4 + NH 3 → (NH 4 )2HPO 4
Safety Precautions for Ammonium Phosphate
Ammonium Phosphate Formula is (NH 4 ) 3 PO 4 . Ammonium Phosphate can be very harmful if swallowed or absorbed. It can raise ammonia levels in your blood, which might harm your organs and lead to symptoms like diarrhea. Plus, touching this chemical can irritate your eyes and skin. So, handle it with care.
To ensure safety when working with Ammonium Phosphate. it is essential to take the following precautions:
- Always wear protective gloves to prevent direct skin contact.
- Utilize specialized protective attire, including safety glasses and masks, when handling the substance.
- After any contact with the chemical, thoroughly wash your hands.
- Clean and wash all safety equipment and clothing before reusing them.
- Avoid inhaling the chemical's fumes or gases.
- These safety measures are crucial to minimize the risks associated with handling Ammonium Phosphate.
Uses of Ammonium Phosphate
Ammonium Phosphate used in several areas:
Fertilizers: Ammonium Phosphate is a key ingredient in some fertilizers, used for plants.
Baking: In the field of baking, Ammonium Phosphate serves as a leavening agent, contributing to the expansion and lightening of baked goods.
Fire Extinguishers: It plays a vital role in dry chemical fire extinguishers, aiding in firefighting efforts.
Ammonium phosphate serves as an efficient fertilizer due to its rapid dissolution and solubility. This compound contains two essential elements, ammonium and phosphate, both of which offer significant benefits to plants.
| Related Links | |
| Barium Bromide Formula | Barium Fluoride Formula |
| Barium Iodide Formula | Hydrobromic Acid Formula |
Ammonium Phosphate Formula FAQs
What is the formula of Ammonium Phosphate?
What is the proper name for ammonium phosphate?
How is Ammonium Phosphate produced?
Is ammonium phosphate utilized in fertilizers?










