
The carbonate ion formula is a polyatomic ion composed of carbon and oxygen atoms. Its chemical formula is CO₃²⁻. This formula represents the arrangement of atoms in the carbonate ion, which plays a significant role in various chemical reactions and is found in a wide range of compounds. In the physiological pH buffering system, bicarbonate plays an important role. It is a polyatomic anion, but it serves as a salt of the acid and a carbon oxoanion.
Structure of Carbonate
As we know the carbonate chemical formula, its structure can be easily drawn on the basis of it. The structure of carbonate ion is the simplest oxocarbon anion. It has D3h molecular symmetry and is made up of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement. It has a total formal charge of 2 and a molecular mass of 60.01 g/mol. The conjugate base of Carbonic acid is the conjugate base of the hydrogen carbonate (bicarbonate) ion.
The carbonate ion has two (long) single bonds to negative oxygen atoms and one short double bond to neutral oxygen in its Lewis structure.Simple, localised Lewis structure of the carbonate ionThis structure contradicts the ion's observed symmetry, which predicts that the three bonds are all the same length and that the three oxygen atoms are all the same. Symmetry can be attained through a resonance among three structures, as in the example of the isoelectronic nitrate ion.
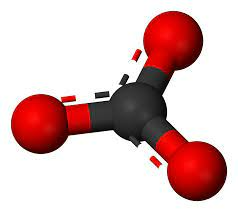
Carbonate Ion Formula
This molecule, whose formula is CO2−3 and commonly known as the carbonate ion, has a trigonal planar molecular structure composed of an atom surrounded by three oxygen atoms. As a Lewis base, it can attract protons in aqueous solutions. It shares similarities with other alkali metal carbonates such as Na2CO3 (also known as washing soda) and Li2CO3, all of which are stable compounds. Lithane, a form of lithium carbonate, was once used to treat manic-depressive individuals. In addition to its chemical uses, carbonate also occurs naturally in limestone and can be converted into sodium bicarbonate (baking soda) or washing soda through heating processes.
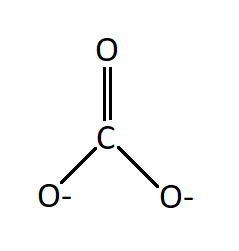
Carbonate Ion Formula and Charge
The carbonate ion (CO₃²⁻) carries a charge of -2. This charge arises from the combination of carbon and oxygen atoms within the ion. Carbon, a non-metal, has a valency of 4, while each oxygen atom has a valency of -2. In the carbonate ion, carbon forms covalent bonds with three oxygen atoms. These bonds result in the distribution of a total of six electrons, with each oxygen atom sharing one electron with carbon. This sharing of electrons contributes to the overall charge of -2 on the carbonate ion.
Carbonate Ion Formula of Acid
The carbonate ion is often involved in the formation of various salts and acids. When the carbonate ion reacts with an acid, it can form a salt and carbon dioxide gas. For example, when carbonic acid (H₂CO₃) reacts with a base or another substance that can accept a proton (H⁺ ion), it dissociates to form the carbonate ion (CO₃²⁻) and water (H₂O):
H₂CO₃ → H₂O + CO₃²⁻
This reaction demonstrates the transformation of carbonic acid into the carbonate ion, highlighting the role of the carbonate ion in acid-base chemistry.
Carbonate Ionic Formula
The carbonate ion's ionic formula, CO₃²⁻, represents its composition and charge as an ion. It's important to note that the carbonate ion is polyatomic, meaning it consists of multiple atoms bound together with an overall charge. This ion is commonly found in various minerals, such as calcite and aragonite. It is a fundamental component in the carbon cycle, playing a crucial role in geological and environmental processes.
| Related Links | |
| Barium Acetate Formula | Barium Nitrate Formula |
| Barium Chloride Formula | Barium Phosphate Formula |
Carbonate Ion Formula FAQs
Is carbonate ionic?
What is the ionic equation for carbonate ions?
Is CO₃²⁻ ionic?










