
CBSE Class 12 Physics Notes Chapter 13: CBSE Class 12 Physics Notes Chapter 13 Nuclei, is the makeup, characteristics, and behaviour of atomic nuclei. A description of the nucleus, including information on protons, neutrons, and nuclear size, opens the section.
Nuclear forces, bound energy, and nuclear stability are all covered in this chapter. It also goes over ideas like the law of radioactive decay, nuclear processes, and mass defect. The chapter also covers half-life and the three types of radioactive decay: beta, gamma, and alpha. The use of nuclear energy is also discussed, along with the operation of nuclear reactors.CBSE Class 12 Physics Notes Chapter 13 Overview
CBSE Class 12 Physics Notes Chapter 13 Nuclei, offers a thorough examination of the atomic nucleus, its elements, and the forces operating within it. The chapter starts out with outlining the protons and neutrons that make up the nucleus's basic structure. It then goes into ideas like nuclear mass, density, and size. It presents nuclear forces, highlighting their small range and function in preserving nuclear stability. The chapter delves into binding energy and mass defect, crucial for understanding why certain nuclei are stable while others undergo radioactive decay. It covers different types of radioactive decay—alpha, beta, and gamma decay—and introduces the concept of half-life, which is vital for understanding the rate of radioactive decay. Furthermore, the chapter discusses nuclear reactions, including fission and fusion, and their applications, particularly in nuclear reactors for energy production. The chapter concludes by highlighting the significant implications of nuclear energy in both peaceful applications and its potential hazards.CBSE Class 12 Physics Notes Chapter 13 PDF
Here we have provided CBSE Class 12 Physics Notes Chapter 13 Nuclei pdf for the ease of students. Students can download this pdf and access it offline.CBSE Class 12 Physics Notes Chapter 13 PDF
CBSE Class 12 Physics Notes Chapter 13 Nuclei
Below we have provided CBSE Class 12 Physics Notes Chapter 13 Nuclei - The nucleus of an atom is a very small area that contains almost all of the mass and all of the positive charge of an atom. Neutrons and protons make up the nucleus. We refer to them as nucleons.Structure of an Atom
The structure of an atom is fundamental to understanding the nature of matter. An atom consists of three primary subatomic particles: protons, neutrons, and electrons, each with distinct properties and locations within the atom.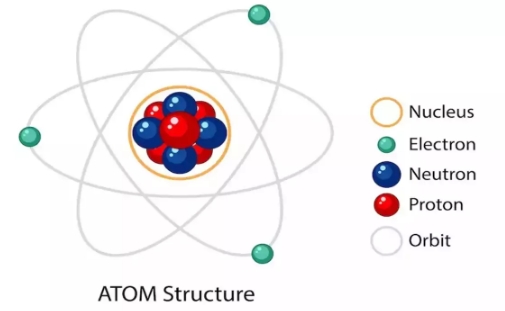 Extranuclear electrons and a core nucleus make up an atom.
Both protons and neutrons can be found in the positively charged nucleus.
Neutrons are neutral, but protons have a positive charge.
Extranuclear electrons and a core nucleus make up an atom.
Both protons and neutrons can be found in the positively charged nucleus.
Neutrons are neutral, but protons have a positive charge.
Nucleus :
Protons : Positively charged particles located in the nucleus. The number of protons determines the atomic number and defines the element.
Neutrons : Neutrally charged particles also located in the nucleus. Neutrons add mass to the atom and contribute to the stability of the nucleus by offsetting the repulsive forces between protons.
Electrons :
Negatively charged particles that orbit the nucleus in specific energy levels or shells. Electrons are much lighter than protons and neutrons and are responsible for the chemical behavior of atoms, including bonding with other atoms.Atomic Number (Z) and Mass Number (A)
The number of protons in the nucleus is indicated by the atomic number (Z). The total amount of protons and neutrons in the nucleus is indicated by the mass number (A). The formula for calculating the number of neutrons (N) is N=A−Z.Nuclear Forces
- Protons and neutrons are held together within the nucleus by nuclear forces.
- These are powerful, yet localised forces.
- They get past the positively charged protons' electrostatic repulsion.
Nuclear Stability
The ability of an atomic nucleus to sustain itself without experiencing spontaneous radioactive decay is known as nuclear stability. The equilibrium between the repellent electromagnetic forces (which push protons away because of their positive charge) and the attracting nuclear forces (which hold protons and neutrons together) determines the stability of a nucleus.- The ratio of protons to neutrons determines the stability of a nucleus.
- Stability is guaranteed for light nuclei (Z<20) with about equal numbers of protons and neutrons.
- The ideal neutron-to-proton ratio rises for heavier nuclei.
Factors Affecting Nuclear Stability
Proton-Neutron Ratio
The stability of a nucleus is largely dependent on the proton to neutron ratio. A ratio of about 1:1 is suitable for lighter elements, but a larger neutron-to-proton ratio is needed for heavier elements in order to balance out the increasing repulsive force between protons.Binding Energy
The amount of energy required to split the nucleus into individual protons and neutrons is known as the binding energy per nucleon. Greater nuclear stability is indicated by a higher binding energy. The most stable nuclei have binding energies that are near to iron-56.Size of the Nucleus
Greater repulsive forces between the protons are found in larger nuclei (which have more protons). Consequently, unstable materials such as uranium or thorium are more prone to undergo radioactive decay in order to become stable.Radioactivity
The spontaneous disintegration of an unstable nucleus is known as radioactive decay. Alpha (α), beta (β), and gamma (γ) decay are the three main forms of radioactive decay. Gamma decay yields high-energy photons, beta decay releases beta particles (electrons or positrons), and alpha decay releases an alpha particle (helium nucleus).Radioactive Decay Law
The Radioactive Decay Law describes the statistical behavior of radioactive substances as they undergo decay over time. It provides a mathematical model for predicting the number of remaining undecayed nuclei in a sample at any given time. N(t)=N0×e(−λt) is the exponential decay law that governs radioactive decay. The number of radioactive nuclei that are still present at time t is denoted by N(t). N₀ represents the starting count of radioactive nuclei, while λ denotes the decay constant.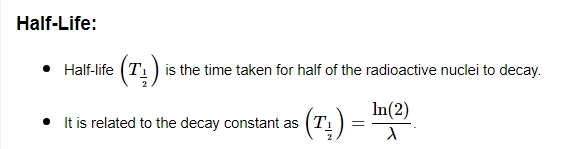
Average Life or Mean Life(τ)
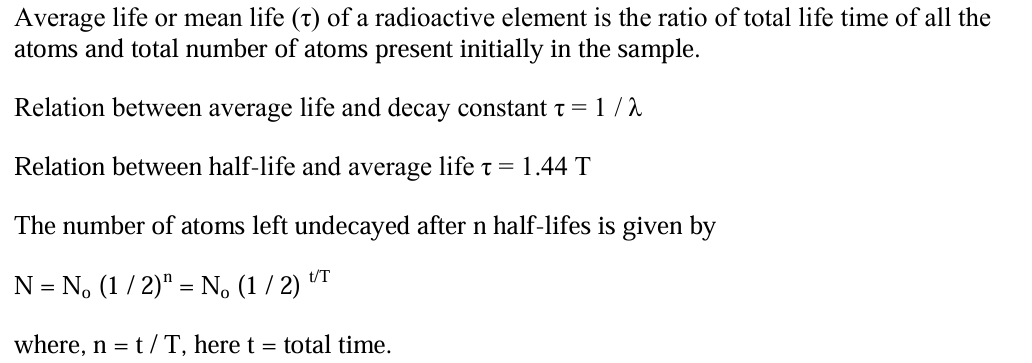
Nuclear Chain Reaction
A nuclear chain reaction is the result of a series of fission reactions that begin when the particle that initiates the reaction is created as a product and continues to participate in the nuclear fission reaction. Nuclear chain reaction are of two types (i) Controlled chain reaction (ii) Uncontrolled chain reactionThermonuclear Energy
Thermonuclear energy is the energy released during nuclear fusion, a process in which atomic nuclei combine to form a heavier nucleus. This fusion process occurs under extremely high temperatures and pressures, similar to the conditions found in the cores of stars, including our sun. Thermonuclear energy is considered a powerful and efficient source of energy, with potential applications in both civilian and military sectors.Nuclear Fission and Fusion
Nuclear fission and fusion are two different nuclear reactions that release a tremendous amount of energy. Both processes are fundamental to nuclear physics and have significant applications in energy production, medicine, and weaponry. Nuclear fission and fusion are powerful processes with significant implications for energy production, medicine, and weaponry. Fission is currently used in nuclear reactors and weapons, while fusion holds promise for the future of clean and virtually limitless energy, though practical fusion power plants are still under development.- A heavy nucleus can split into two lighter nuclei through a process known as nuclear fission.
- Two light nuclei can combine to generate a heavier nucleus through a process called nuclear fusion.
- A tremendous quantity of energy is released during both fission and fusion.
Nuclear Reactors
- Nuclear reactors produce energy by means of regulated fission processes.
- They are made up of a coolant, control rods, moderators, and nuclear fuel.
- To control the reaction, surplus neutrons are absorbed by control rods.
Working Principle of a Nuclear Reactor:
Fission Reaction :
The nuclear fuel is bombarded with neutrons, which causes the atoms' nuclei to split (fission), releasing a significant quantity of energy in the form of heat and more neutrons.Chain Reaction :
A self-sufficient chain reaction can be started by the neutrons generated by fission in other nuclei. This chain reaction is controlled by the control rods, which guarantee its stability.Heat Transfer :
The heat generated in the reactor core is absorbed by the coolant, which then either boils to produce steam directly or transfers heat to a secondary loop where water is converted to steam.Electricity Generation :
The steam produced drives a turbine connected to an electric generator, converting thermal energy into electrical energy.Atoms Nucleus
The tiny, dense area in the middle of an atom is called the atomic nucleus. The Geiger-Marsden gold foil experiment, carried out in 1909, allowed Ernest Rutherford to discover the atomic nucleus in 1911. Protons and neutrons make up the positively charged nucleus of an atom. A cloud of negatively charged electrons envelops the nucleus. Electrostatic force holds the nucleus and electron cloud together. The nucleus of an atom contains the majority of its mass. The atom's total mass is mostly determined by the electron cloud. The nuclear force holds the protons and neutrons in the nucleus together.Benefits of CBSE Class 12 Physics Notes Chapter 13
The benefits of studying Chapter 13 "Nuclei" in CBSE Class 12 Physics notes are substantial, providing students with a comprehensive understanding of nuclear physics and its applications. Here are some key benefits:Strong Conceptual Foundation : The chapter provides a detailed explanation of nuclear structure, forces, and reactions, which are fundamental concepts in modern physics and essential for understanding advanced topics in quantum mechanics and nuclear engineering.
Exam Preparation : The well-organized notes help students efficiently review key topics, enhancing their ability to recall information during exams, which is crucial for scoring well in board exams.
Problem-Solving Skills : The chapter includes numerical problems related to binding energy, radioactive decay, and nuclear reactions, helping students develop critical problem-solving skills that are important for both board exams and competitive exams.
Real-World Applications : Understanding nuclear physics has practical applications in various fields, including medical imaging, cancer treatment (radiotherapy), energy production, and even astrophysics, making the knowledge gained from this chapter highly relevant and applicable.
Foundation for Higher Education : A solid understanding of nuclear physics is essential for students who plan to pursue higher education in fields like physics, engineering, medicine, and environmental science.
CBSE Class 12 Physics Notes Chapter 13 FAQs
What are the characteristics of nuclei Class 12?
What is the size of the nucleus?
What is the structure of atoms and nuclei notes?
What are the basic properties of nuclei?










