
CBSE Class 12 Physics Notes Chapter 12: In "Atoms," Chapter 12 of CBSE Class 12 Physics, the structure of atoms and the development of atomic models are examined. The early atomic hypotheses, such as Dalton's model, are covered in the first section of the chapter before J.J.
Thomson's discovery of the electron. The gold foil experiment by Rutherford, which produced the nuclear model of the atom and highlighted the nucleus and orbiting electrons, is then covered. Along with a brief overview of X-rays and the dual nature of matter, the chapter also covers energy levels, spectral lines, and the Bohr model of the hydrogen atom.CBSE Class 12 Physics Notes Chapter 12 Overview
CBSE Class 12 Physics Notes Chapter 12 Atoms, explores the concept of atomic structure by following the evolution of atomic models across time. Early theories, like Dalton's atomic theory, which defined atoms as indivisible particles, was where it all started. The discovery of the electron by J.J. Thomson and his "plum pudding" concept, in which electrons were lodged in a positively charged sphere, come next. The gold foil experiment by Rutherford, which refuted Thomson's theory and gave rise to the nuclear model of the atom, is the subject of the next section of the chapter. According to Rutherford's hypothesis, an atom is made up of a compact, positively charged nucleus that is encircled by electrons in a vacuum. The Bohr model was developed as a result of this concept's inability to explain why atoms are stable. The stability of atoms and the discrete spectral lines seen in atomic spectra were explained by Niels Bohr's model, which linked quantised electron orbits with Rutherford's nuclear structure. The chapter also discusses the duality of matter and light, including a brief overview of wave-particle duality and de Broglie's theory.CBSE Class 12 Physics Notes Chapter 12 PDF
This chapter provides a foundational understanding of atomic theory, essential for further studies in quantum mechanics and modern physics. Hence here we have provided CBSE Class 12 Physics Notes Chapter 12 Atoms pdf for the ease of the students -CBSE Class 12 Physics Notes Chapter 12 PDF
CBSE Class 12 Physics Notes Chapter 12 Atoms
Here we have provided CBSE Class 12 Physics Notes Chapter 12 Atoms -Nucleus
The nucleus is the small, dense central core of an atom, containing most of the atom's mass. It is composed of protons, which carry a positive charge, and neutrons, which are electrically neutral. Together, these particles are known as nucleons.
Atomic Mass Unit
The atomic mass unit (amu), also known as the unified atomic mass unit (u) or Dalton (Da), is a standard unit of mass that quantifies mass on an atomic or molecular scale. It is defined as one-twelfth of the mass of an unbound carbon-12 atom, which is in its ground state and at rest.
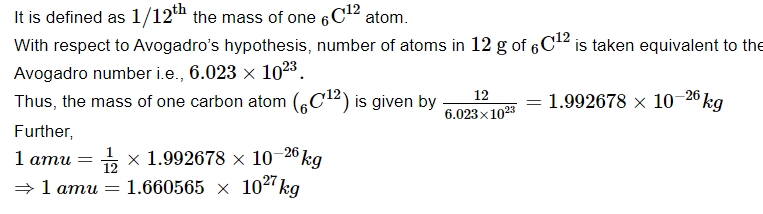
Energy Equivalent of Atomic Mass Unit
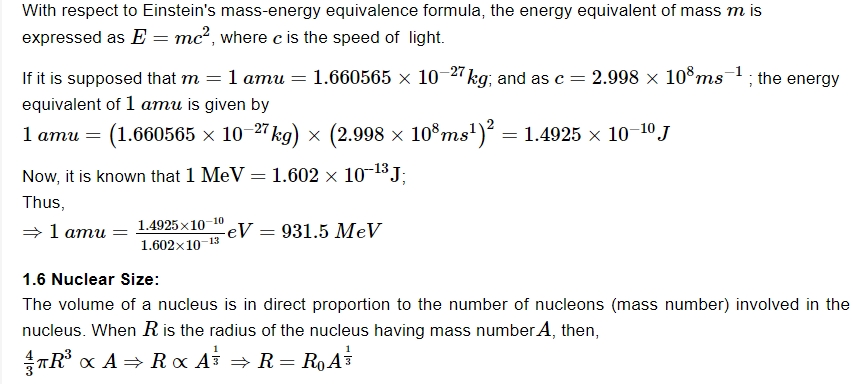
Nuclear Density
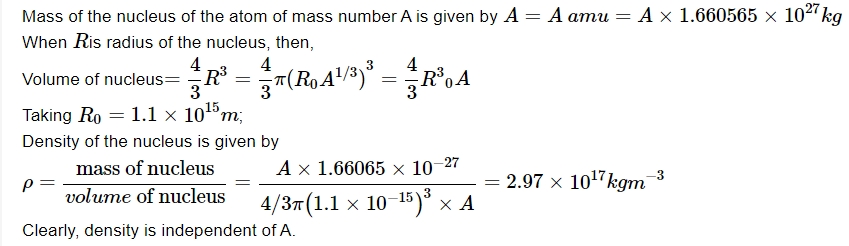
Mass Defect

Binding Energy Per Nucleon
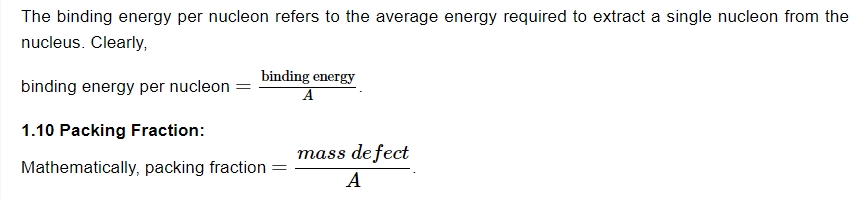
Natural Radioactivity
Natural radioactivity refers to the spontaneous emission of particles or electromagnetic radiation from the unstable nuclei of certain naturally occurring elements. This process is a form of nuclear decay, where the unstable atomic nuclei transform into more stable configurations by emitting radiation.Laws of Radioactivity Decay
The laws of radioactive decay describe how unstable atomic nuclei lose energy by emitting radiation. These laws are fundamental to understanding the behavior of radioactive materials over time.

In this case, the radioactive sample's decay constant is denoted by the constant of proportionality λ . It is also known as the transformation constant or the disintegration constant. The radioactive sample's nature determines its value. Furthermore, the expression's negative sign indicates that the sample's atomic count decreases over time.

Half Life
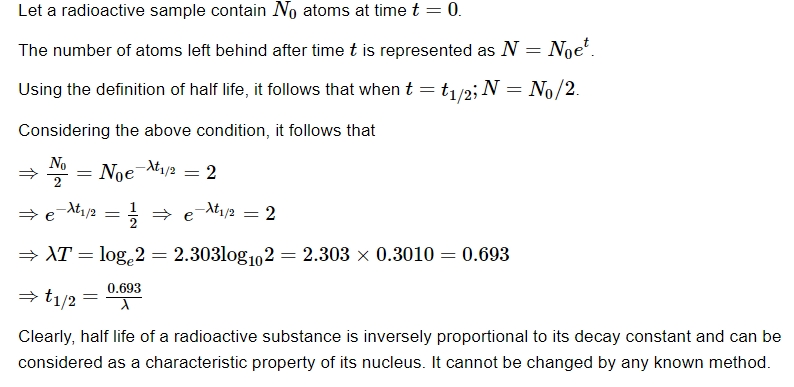
Mean Life or Average Life
The average life of a radioactive substance refers to the average time for which the nuclei of the atoms of the radioactive substance exist. It is mathematically given by
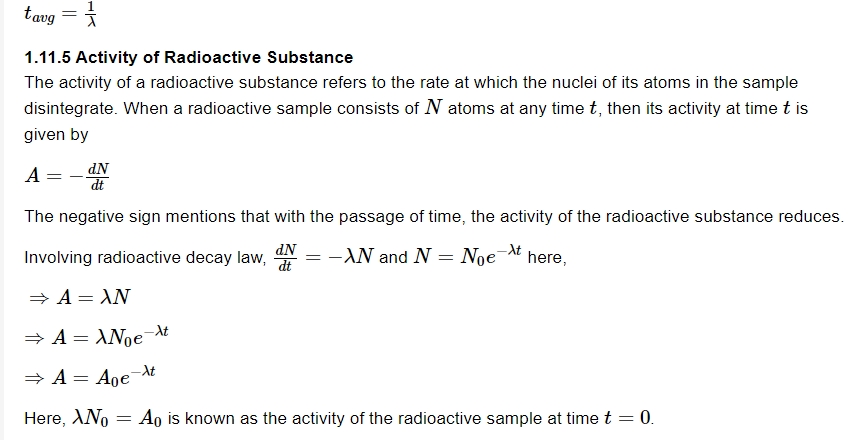
Units of Activity
The activity of a radioactive sample can be expressed as the number of disintegrations per second. The practical unit of activity of a radioactive sample is curie (Ci).
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a sustained nuclear chain reaction, primarily for generating electricity, producing isotopes for medical and industrial use, or for research purposes. It operates on the principle of nuclear fission, where the nucleus of a heavy atom, such as uranium-235 or plutonium-239, splits into smaller nuclei when struck by a neutron, releasing a large amount of energy.Key Components of a Nuclear Reactor:
Fuel :
The primary fuel used in a nuclear reactor is enriched uranium or plutonium, which undergoes fission to release energy.Moderator :
The moderator slows down the neutrons produced during fission, increasing the likelihood of further fission events. Common moderators include water, heavy water, and graphite.Control Rods :
Control rods are made of materials like boron, cadmium, or hafnium, which absorb neutrons. They are inserted or withdrawn from the reactor core to control the rate of the nuclear reaction.Coolant :
The coolant transfers heat from the reactor core to a steam generator or directly to a turbine. Common coolants include water, heavy water, gas, or liquid metal.Reactor Core :
The reactor core contains the fuel assemblies, moderator, and control rods, where the fission reaction takes place.Containment Structure :
A robust structure that encloses the reactor core to prevent the release of radioactive materials in case of an accident.Free Electrons in Metals
One could think of electrons as the building blocks of an atom. Electrons in a metal are free to roam freely and arbitrarily through the atomic gaps. On the other hand, the metal's surface produces an equal positive charge as soon as an electron leaves the metal. The electron is subsequently drawn back into the metal and stays within it as a result. It is noted that the pull on the electrons at the surface depends on the properties of the metal surface and is represented by a property of the metal known as the work function.Work Function
The phrase "work function" refers to the minimal amount of energy required for an electron to simply exit a metal surface. Electron emission is the term for this process, which can be accomplished in the following ways: Thermionic emission: Heat is produced as a result of the additional energy. We refer to the released electrons as thermo-electrons. Photoelectric emission: In this case, electromagnetic radiation provides the additional energy. We refer to the released electrons as photoelectrons. Secondary emission: In this instance, the rapidly travelling electrons release secondary electrons when they collide with the metal surface. Field emission: In this case, electrostatic fields are used to help release electrons.Thomson’s Atom Model
According to this idea, electrons are contained within the sphere of positive charges, which are uniformly distributed throughout the sphere, much like plums or seeds in custard or watermelons. For this reason, the plum-pudding model is another name for Thomson's atom model. Every atom is electrically neutral because the total positive charge within it equals the total negative charge carried by its electrons. When an atom is slightly perturbed, its electrons fluctuate around their equilibrium positions, which causes radiation of specific frequencies to be ejected in the form of ultraviolet, visible, or infrared light.Failure of Thomson’s Atom Model
It was necessary to abandon this model for the following reasons: It was unable to convey the spectral lines' origin in a series, as was the case with hydrogen atoms. The experiment was unable to accurately depict the dispersion of α particles at large angles, unlike Rutherford's α scattering experiment.Rutherford’s Atomic Model
Carrying forward the Gieger-Marsden experiment was Rutherford. According to this idea, the atom is composed of a small, dense, positively charged core known as the nucleus, where almost all of the mass is concentrated. Similar to how planets orbit the Sun, the light-weight component, or electrons, revolves around it.Impact Parameter
When the alpha particle (Helium) is far from the atom's nucleus, the impact parameter (b) is the initial velocity vector's perpendicular distance from the nucleus's central line. It uses this formula: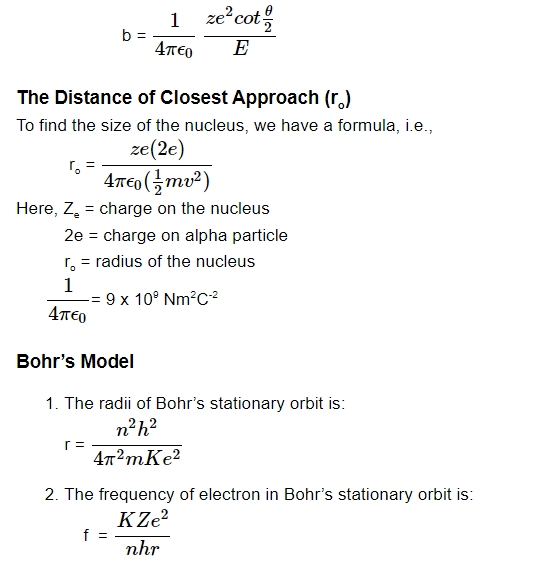
Benefits of CBSE Class 12 Physics Notes Chapter 12
The benefits of studying Chapter 12 "Atoms" in CBSE Class 12 Physics notes are numerous, offering a strong foundation in understanding atomic structure and related concepts. Here are some key benefits:Conceptual Clarity : The notes provide a clear explanation of complex concepts such as atomic models, the discovery of the electron, and the nucleus, helping students grasp the fundamental ideas of atomic physics.
Historical Perspective : The chapter traces the evolution of atomic theory, giving students a deeper appreciation of how scientific models are developed and refined over time.
Exam Preparation : Well-organized notes help in efficient revision, making it easier to recall key concepts and theories during exams, thus enhancing performance.
Application of Concepts : Understanding the atomic structure is crucial for advanced topics in physics, chemistry, and even biology. The chapter lays the groundwork for further studies in quantum mechanics and nuclear physics.
Problem-Solving Skills : The chapter includes numerical problems related to atomic models and spectra, helping students develop problem-solving techniques that are essential for scoring well in exams.
CBSE Class 12 Physics Notes Chapter 12 FAQs
What are atoms mostly made of?
Do atoms build matter?
What is the purpose of atoms?
Are CBSE Class 12 Physics Notes Chapter 12 Atoms helpful?
Can I access CBSE Class 12 Physics Notes Chapter 12 Atoms offline?










