
CBSE Class 12 Physics Notes Chapter 11: The idea that particles, such as electrons, display both wave-like and particle-like qualities is explored in Chapter 11 of CBSE Class 12 Physics, "Dual Nature of Matter and Radiation," and is a fundamental idea in quantum mechanics. Important experiments like the photoelectric effect, which demonstrates that light is a particle, and de Broglie's hypothesis, which proposes that particles like electrons have a wavelength in common, are covered in this chapter.
For an understanding of atomic and subatomic processes, this concept of duality is essential. The chapter also discusses how these events have affected the development of quantum theory and how it is used in modern physics.CBSE Class 12 Physics Notes Chapter 11 Overview
"Dual Nature of Matter and Radiation," the 11th chapter of CBSE Class 12 Physics, explores the revolutionary concept that matter and radiation have dual properties, acting as both particles and waves. The comprehension of the quantum mechanical perspective on the physical universe begins with this chapter. The study of the photoelectric effect, an experiment carried out by Heinrich Hertz and subsequently clarified by Albert Einstein, opens the chapter. It was shown via this result that light, which is usually thought of as a wave, can also have particle-like characteristics. Specifically, photons, which are able to expel electrons from a metal surface, are particles. Einstein was awarded the Nobel Prize for his explanation of the photoelectric effect, which also established that light is a particle. The de Broglie theory, which states that particles like electrons also have a wave character and that their momentum is correlated with their wavelength, is then introduced in the chapter. Electron diffraction experiments, in which electrons behave like waves, provide more evidence for this wave-particle duality.CBSE Class 12 Physics Notes Chapter 11 PDF
CBSE Class 12 Physics Notes Chapter 11 is crucial for students to understand the principles underlying quantum mechanics and the behavior of particles at atomic and subatomic scales, bridging classical and modern physics. Here we have provided CBSE Class 12 Physics Notes Chapter 11 pdf -CBSE Class 12 Physics Notes Chapter 11 PDF
CBSE Class 12 Physics Notes Chapter 11 Dual Nature of Matter and Radiation
Here we have provided CBSE Class 12 Physics Notes Chapter 11 Dual Nature of Matter and Radiation - The idea that particles like electrons and electromagnetic radiation like light have both particle and wave-like characteristics is known as the "dual nature of matter and radiation." This basic principle of quantum mechanics has been experimentally verified by a number of phenomena.Cathode Rays
A cathode ray is an atom's stream of rapidly moving electrons. They can be generated at low pressures, less than 0.001 mm of a mercury column, in a discharge tube.Properties of Cathode Rays:
- It's not electromagnetic radiation here.
- The electric and magnetic fields deflect these beams.
- Heat is produced by the metals when they are exposed to cathode rays.
- The thin gold or aluminium foils are easily penetrated by cathode rays without the necessity for puncturing.
- Any kind of physical or chemical alteration can occur to the cathode rays.
- These rays have the power to cast shadows on objects as they move quickly and energetically in a straight path.
- When they strike an object with a high atomic weight, they can generate X-rays.
- They have an impact on the photographic plate because certain compounds exhibit phosphorescence and fluorescence when exposed to cathode rays.
- An electron has a particular charge of 1.7589 x 1011 C / kg.
Positive Rays
Positive rays were found by scientists Goldstein. They are referred to as the gas filled in the discharge tube's moving positive ions. The mass of the gas's atoms and these particles are the same.Properties:
- Positively charged particles that move quickly make up the positive rays.
- In both electric and magnetic fields, these rays are deflected.
- Only a straight path will allow these rays to travel.
- The cathode rays travel at a faster speed than the positive rays.
- Fluorescence and phosphorescence can be produced by positive photons.
Electron Emission
The work function of a metal is the least amount of energy needed by an electron to leave its surface. It is measured in electron volts (eV), and is represented by the symbol o. One electron volt(eV) is defined as the energy that the electron gains for accelerating by a potential difference of 1 volt. We can say that 1 eV = 1.602 × 10-19 J. The value of the work function is dependent on the properties of the metal and the nature of its surface. Electron emission is the process by which electrons escape from a metal's surface. It comes from the three procedures listed below. Thermionic: Heat transfers enough thermal energy to liberate electrons to allow them to separate off the metal surface. Photoelectric emission is the release of a metal surface's electrons when it is exposed to light at the right frequency. We refer to these electrons as photoelectrons. Field emission: Electrons can be extracted from metal by subjecting it to an electric field.Read More - Strategy to Solve Class 12 Sample Papers Efficiently
Photoelectric Effect
The photoelectric effect is a phenomenon in physics where light, when shone onto a material (typically a metal), causes the emission of electrons from the surface of that material. This effect provided crucial evidence for the quantum nature of light and contributed significantly to the development of quantum mechanics.Hertz’s observations
Heinrich Hertz made the discovery of photoelectric emission in 1887 while conducting research on electromagnetic waves. He noticed that when light passes through a metal detector loop, the electrons on the surface gain enough energy to displace the positive ions' attraction force and exit into the surrounding area.Observation by Hallwach and Lenard
Through their research, scientists Hallwachs and Lenard found that there is a minimal cut-off frequency below which electrons cannot be released. The threshold frequency is thought to be at this frequency. Certain metals, such as zinc (Zn), cadmium (Cd), magnesium (Mg), etc., have been found to react to short-wavelength UV radiation by releasing electrons from their surfaces. Lithium (Li), sodium (Na), potassium (P), caesium (Cs), and rubidium (Rb) are among the alkali metals that are light-sensitive.Photoelectric Effect- Experimental Study
The experimental setup consists of a metal plate (collector) and a photosensitive plate (emitter) attached to an evacuated glass/quartz tube. In the absence of air resistance, this tube will permit electrons to go from the emitter to the collector. The potential difference (V) between the two plates is sustained by the battery. The commutator has the ability to reverse the polarity.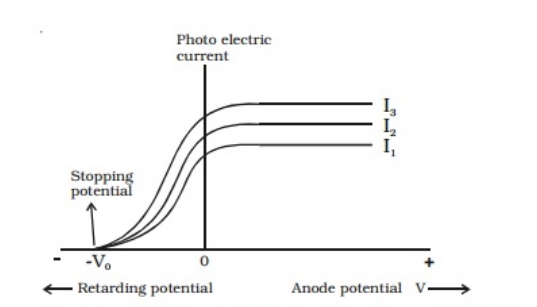 Electrons are released and visible light is absorbed by the photosensitive plate, also known as the emitter. All of the electrons that are released by the emitter are collected by the metal plate (collector). A photoelectric current flow results from this (from emitter to collector). This is consistently in opposition to the electrons' directions. A voltmeter and current probe can be used to measure the potential difference (V) and current (I) between the plates.
Electrons are released and visible light is absorbed by the photosensitive plate, also known as the emitter. All of the electrons that are released by the emitter are collected by the metal plate (collector). A photoelectric current flow results from this (from emitter to collector). This is consistently in opposition to the electrons' directions. A voltmeter and current probe can be used to measure the potential difference (V) and current (I) between the plates.
Einstein’s Photoelectric Equation
The idea put forth by Albert Einstein is that radiation energy is composed of distinct units. According to him, photoelectric emission is not produced by a continuous absorption of radiation energy. These discrete units were given the term radiation energy quanta by him. Planck's constant, h, and the frequency of light, represent each quantum of energy.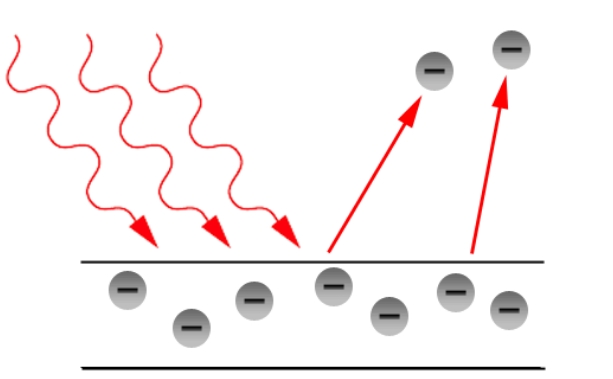 Therefore, Einstein’s photoelectric equation is given as
Ek =h–o or
Ek =h–ho or
Ek =h(–o)
Particle nature of light:
The photoelectric effect provides proof that light behaves as quanta, or energy packets with an h-value, when it interacts with matter. The particles' motion and energy have distinct values. A photon was identified as this particle.
Therefore, Einstein’s photoelectric equation is given as
Ek =h–o or
Ek =h–ho or
Ek =h(–o)
Particle nature of light:
The photoelectric effect provides proof that light behaves as quanta, or energy packets with an h-value, when it interacts with matter. The particles' motion and energy have distinct values. A photon was identified as this particle.
- The radiation incident behaves as though it is composed of photons.
- Every photon has a momentum of p (= h/c) and an energy of E=h, where c is the speed of light.
- Photons have no electrical charge. There are no magnetic or electric fields that can deflect them.
- Both total momentum and total energy are unaltered in a collision between photons and particles.
Wave nature of Matter:
The wave nature of matter is a concept in quantum mechanics that suggests that all particles exhibit both wave-like and particle-like properties. This idea was first proposed by the French physicist Louis de Broglie in 1924 and is a fundamental aspect of quantum theory. It was proposed by de Broglie. The wavelength is given as = hp=hm where p is the momentum. Davisson-Germer Experiment: In 1927, Davisson and Germer established the material particle's de Broglie's wave character; GP Thomson confirmed this same fact in 1928. This experiment has been verified using Ni crystal. Davisson and Germer discovered that at various scattering angles, the electron beam's intensity varies. At a potential difference of 54 V, it reaches its maximum for a 50° diffraction angle. The wavelength is specified as = 12.27VAThe Photoelectric Cell
The photoelectric effect is the basis for how a photoelectric cell operates. Light affects the electrical characteristics of the gadget. Another name for it is the electric eye. They come in three varieties: photovoltaic, photo emissive, and photoconductive.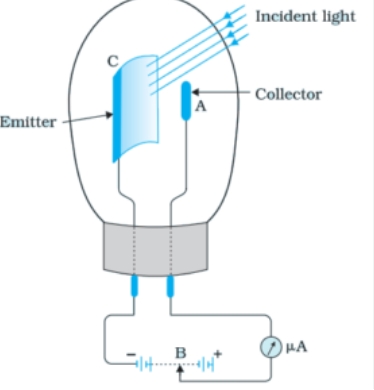 Photocells are used in television cameras to capture and broadcast scenes as well as to replicate sound in motion images. Photoelectric cells are used in the manufacturing sector to find microscopic imperfections in metal sheets.
Photocells are used in television cameras to capture and broadcast scenes as well as to replicate sound in motion images. Photoelectric cells are used in the manufacturing sector to find microscopic imperfections in metal sheets.
Working Principle :
- When light of a certain frequency (above the threshold frequency) shines on the cathode, it excites the electrons in the material, causing them to be ejected from the surface.
- These emitted electrons are then attracted to the positively charged anode, creating a flow of electric current through the circuit connected to the photoelectric cell.
- The magnitude of the current is proportional to the intensity of the incident light, allowing the photoelectric cell to measure light intensity or control a circuit based on light conditions.
Applications:
Light Meters : Used in photography to measure the intensity of light and determine the correct exposure settings.
Automatic Lighting : Common in streetlights that automatically turn on at dusk and off at dawn.
Solar Panels : Convert sunlight directly into electrical energy, using the principles of the photoelectric effect.
Safety Devices : Used in burglar alarms and other security systems to detect interruptions in a light beam.
Benefits of CBSE Class 12 Physics Notes Chapter 11
The benefits of studying Chapter 11 "Dual Nature of Matter and Radiation" in CBSE Class 12 Physics include:Foundation in Quantum Mechanics : This chapter provides a fundamental understanding of quantum mechanics, a critical area in modern physics. It introduces key concepts like wave-particle duality, essential for further studies in physics and related fields.
Conceptual Clarity : The chapter helps students grasp complex ideas like the photoelectric effect, de Broglie's hypothesis, and Heisenberg's Uncertainty Principle. This clarity is crucial for performing well in exams and developing a strong foundation for higher education.
Application-Oriented Learning : Understanding the dual nature of matter and radiation has practical applications in technologies like semiconductors, lasers, and electron microscopes. This knowledge is vital for students pursuing careers in engineering, technology, and research.
Exam Preparation : Comprehensive notes on this chapter help in effective revision and preparation for board exams. The chapter often features in competitive exams like JEE and NEET, making it a high-scoring topic.
Critical Thinking Development : The concepts in this chapter encourage students to think critically and question classical physics ideas, fostering a deeper understanding of the physical world.
CBSE Class 12 Physics Notes Chapter 11 FAQs
How do matter and radiation have a dual nature?
What is the dual nature of radiation and matter important?
What is another name for the dual nature of radiation and matter?
What is the dual behaviour of matter and radiation?










