Chromium VI Oxide Formula: The chemical formula for Chromium(VI) oxide is CrO 3 . Chromium, symbolized as Cr with an atomic number of 24, is a metallic element characterized by its lustrous, steely grey appearance and hardness. Positioned in the sixth group of the periodic table, Chromium exhibits a remarkable resistance to corrosion. When introduced into steel, it enhances the steel's resistance to corrosion, resulting in the formation of stainless steel. In the 19th century, Chromium found extensive use as a component in various paints due to its reflective properties when polished.
An oxide, often referred to as a dianion of oxygen, is a chemical compound composed of at least one oxygen atom and one other element as indicated by its chemical formula. Oxygen, a highly reactive nonmetal with an atomic number of 8, is both colorless and odorless and stands as the most abundant element on Earth. Oxygen plays a critical role in several industries, including textiles, plastics, submarines, and space flights.
Chromium VI Oxide Formula
Chromium(VI) oxide, alternatively known as chromium trioxide or chromic anhydride, is a potent oxidizer. This compound typically exists as a dark red granular solid but takes on a dark purple hue in its anhydrous state. Comprising chromium and oxygen, its chemical formula is CrO 3 . In the following sections, we will explore its structure, preparation, chemical and physical properties, as well as its essential applications.
Chromium VI Oxide Formula Structure
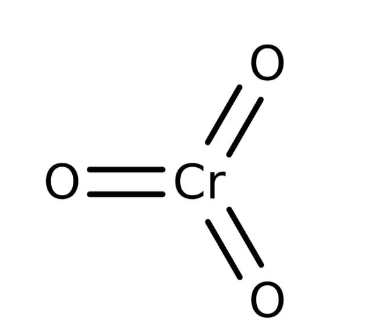
Regarding its structure, Chromium(VI) oxide boasts a chemical formula of CrO 3 , with a molar mass of 99.999 g/mol. In its molecular structure, one Cr 6+ atom forms three bonds with oxygen atoms.
Chromium VI Oxide Formula Physical Properties
Chemical Formula: Chromium VI Oxide Formula is CrO 3
Appearance: Dark red granular solid
Odor: Odorless
Solubility: It is soluble in water, sulfuric acid, nitric acid, acetic acid, and acetone.
Melting Point: 197°C
Boiling Point: 250°C
Density: 2.7 g/cm3
Molar Mass: 99.993 g/mol
Chromium VI Oxide Formula Chemical Properties
Chromium trioxide undergoes decomposition at temperatures above 197°C, releasing oxygen and forming Cr 2 O 3 .
4CrO 3 → 2Cr 2 O 3 + 3O
When Chromium(VI) oxide reacts with water, it forms chromic acid, which has the chemical formula H 2 CrO 4 and is considered a weak acid.
CrO 3 + H 2 O → H 2 CrO 4
Uses of Chromium VI Oxide
It finds use in the production of dyes, inks, and paints.
Chromium(VI) oxide plays a significant role in chrome plating. When it reacts with metals such as zinc and cadmium, it forms a protective, corrosion-resistant layer.
Its applications extend to tanning and engraving processes.
It is used in aluminum anodizing, enhancing the protective coatings on aluminum surfaces.
Chromium(VI) oxide is utilized in aerospace industries for various purposes.
In the field of photography, it serves specific functions.
It acts as an oxidizing agent in diverse chemical processes.
Chromium(VI) oxide, with its chemical formula CrO3, is a versatile and powerful compound. It plays a crucial role in a variety of applications, such as the production of dyes, inks, and paints, contributing to vibrant colors and visual appeal. It is also pivotal in chrome plating, providing corrosion resistance to various metals. Moreover, it finds utility in tanning, engraving, and enhancing the protective coatings on aluminum surfaces through anodizing.
The aerospace industry benefits from its use in surface treatments and coatings, ensuring the longevity of components exposed to challenging conditions. In photography, Chromium(VI) oxide plays a significant role in photographic development processes, contributing to the art of capturing and preserving visual memories.
Lastly, as an oxidizing agent, Chromium(VI) oxide is indispensable in a wide range of chemical reactions, making it a versatile compound for researchers and chemists. This compound's unique properties and diverse applications underscore its importance across various fields, from art and industry to technology and science.
| Related Links | |
| Hydroiodic Acid Formula | Nitrogen Dioxide Formula |
| Hydrobromous Acid Formula | Hyposulfurous Acid Formula |
Chromium VI Oxide Formula FAQs
What is the chemical formula for Chromium(VI) oxide?
What is the appearance of Chromium(VI) oxide?
What is the melting point of Chromium(VI) oxide?
What are the essential uses of Chromium(VI) oxide?