
Arsenic Acid Formula: Arsenic acid, also known as arsoric acid, Holds the chemical formula H 3 AsO 4 . To provide a more detailed description, it can be represented as AsO(OH) 3 . This Colorless acid serves as the arsenic counterpart to phosphoric acid and shares similar behaviors with arsenate and phosphate salts. Arsenic acid is typically not isolated in its pure form but rather exists in solution, where it primarily exists in an ionized state. It's hemihydrate form, 2H 3 AsO 4 ·H 2 O, can form stable crystalline structures, although these samples will lose water through dehydration when exposed to temperatures of around 100 °C.
Arsenic is a chemical element denoted by the symbol As, and it holds the atomic number 33. This naturally occurring element is widespread, being present in rocks, soil, water, air, as well as in plants and animals. Arsenic is classified as a metalloid and exists in various allotropes, with its gray form being of particular significance for industrial applications.
Arsenic Acid Formula
Arsenic acid is an oxoacid of arsenic, featuring one oxo group and three hydroxy groups bonded to a central arsenic atom. It acts as the conjugate acid of an arsenate ion (1-) and an arsenate ion. In its liquid state, arsenic acid appears as a clear and colorless aqueous solution and is noncombustible. In its solid form, it appears as white crystals and is also referred to as Orthoarsenic acid. Arsenic acid finds utility as a wood preservative, a finishing agent for glass and metal, and as a reagent in the synthesis of various dyestuffs and organic arsenic compounds.
The chemical formula for arsenic acid, also known as the arsenic acid formula, is elucidated in this text. Arsenic shares its five electrons with four oxygen atoms. Three of these oxygen atoms attain electron completeness by sharing electrons with three hydrogen atoms. As a result, arsenic acid comprises arsenic, four oxygen, and three hydrogen atoms. The atomic mass of arsenic acid is calculated at 141.94 g/mol.
Arsenic Acid Formula Structure
Arsenic acid formula is H 3 AsO 4 . Arsenic acid is an oxoacid of arsenic characterized by the presence of one oxo group and three hydroxy groups, all bonded to the central arsenic atom. This compound exists as a clear, colorless solution in its liquid form and as white, translucent crystals in its solid state. Notably, arsenic acid is hygroscopic and non-combustible.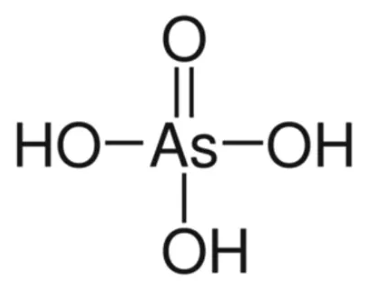
Preparation of Arsenic Acid
Arsenic acid can be synthesized by reacting arsenic trioxide with concentrated nitric acid (HNO₃). This reaction also produces dinitrogen trioxide as a byproduct:
As 2 O 3 + 2HNO 3 + 2H 2 O → 2H 3 AsO 4 + N 2 O 3
Subsequently, the resulting solution can be cooled to yield colorless crystals of the hemihydrate H 3 AsO 4 ·1⁄2H 2 O. Alternatively, when crystallization occurs at lower temperatures, the dihydrate form H 3 AsO 4 ·2H 2 O is produced.
Arsenic Acid Formula Physical Properties
Arsenic acid exhibits distinct physical properties:
In its liquid state, it takes the form of a colorless aqueous solution.
When solidified, it appears as white crystalline material.
Arsenic acid is highly soluble in water.
Its melting point is 35.5 °C.
Its boiling point is 120 °C.
Arsenic Acid Formula Chemical Properties
Arsenic can undergo a chemical reaction with three molecules of water and ozone to produce arsenic acid:
2As + 3H 2 O + 5O 3 → 2H 3 AsO 4 + 5O 2Uses of Arsenic Acid
Arsenic acid finds various practical uses in different industries:
It serves as a reagent in the synthesis of certain dyes.
Arsenic acid is used as a finishing agent in the manufacturing of metal and glass products.
It is utilized in wood preservatives.
Arsenic acid also functions as a broad-spectrum biocide.
Safety
Arsenic acid is highly hazardous and carcinogenic, much like all compounds containing arsenic. Additionally, it exhibits corrosive properties. For instance, its LD50 in rabbits stands at 6 mg/kg (0.006 g/kg).
| Related Links | |
| Hypophosphoric Acid Formula | Sodium peroxide Formula |
| Bromic Acid Formula | Sodium Nitride Formula |
Arsenic Acid Formula FAQs
What is the chemical formula for arsenic acid?
What is the structure of arsenic acid?
What are the primary uses of arsenic acid?
How is arsenic acid prepared?
Is arsenic acid safe to handle?










