
Dimethylglyoxime , also known as DMG, is a white powder with low solubility in water but high solubility in sodium hydroxide or methanol. Its chemical formula is C 4 H 8 N 2 O 2 . and it is commonly referred to as 2,3-Butanedione dioxide. This chemical has various derivatives, which are used as analytical chemistry reagents.
One of its main uses is for detecting nickel ions, but it can also identify other metal ions. In its anionic form, it is called DMGH, while the neutral form is referred to as DMGH2 (with H representing hydrogen). DMGH2 is specifically helpful in identifying nickel and palladium. Additionally, this compound acts as a bidentate ligand with two donor arms.
General Properties of Dimethylglyoxime – C 4 H 8 N 2 O 2
Although generally used as a reagent specific to nickel, Dimethylglyoxime can actually be used to detect other metal ions as well. Many derivatives of Dimethylglyoxime have been synthesized.
It is also known as 2,3-Butanedione, Biacetyl dioxime, dioxime, N-(3-hydroxyiminobutan-2-ylidene) hydroxylamine, etc.
| C4H8N2O2 | Dimethylglyoxime |
| Density | 1.37 g/cm³ |
| Molecular Weight/ Molar Mass | 116.12 g/mol |
| Boiling Point | Not determined |
| Melting Point | 240 to 241 °C |
| Chemical Formula | CH 3 C(NOH)C(NOH)CH 3 |
Dimethylglyoxime Structure – C 4 H 8 N 2 O 2
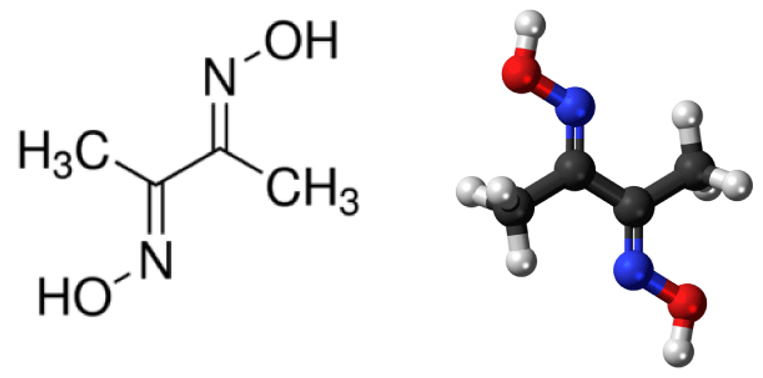
Dimethylglyoxime, also known as DMG, is a white powder that differs in solubility depending on the substance. While it is less soluble in water, it can dissolve in sodium hydroxide solution and methanol. Its chemical formula is C4H8N2O2 and it is also referred to as 2,3-Butanedione dioxide. In analytical chemistry, DMG serves as a reagent and has numerous synthesized derivatives. Besides detecting nickel, it also aids in identifying other metal ions. The anionic form of DMG is called DMGH while the neutral form is DMGH2 (H representing hydrogen). As a bidentate ligand with two donor arms, DMGH2 plays a crucial role in detecting nickel and palladium.
Also Check – Bond Order Formula
Dimethylglyoximato Ligand
In dimethylglyoximato, the charge is -1, which makes it an anionic ligand. The number of donor atoms is 2, which makes it a bidentate ligand. It coordinates through 2 N atoms.
Physical Properties of Dimethylglyoxime – C 4 H 8 N 2 O 2
| Odour | Odourless |
| Appearance | Off White Powder |
| Complexity | 112 |
| Dipole moment | 0 |
| Hydrogen Bond Donor | 2 |
| Solubility | Insoluble in water, soluble in alcohol |
Also Read – Aluminium Nitrate Formula
Chemical Properties of Dimethylglyoxime – C 4 H 8 N 2 O 2
Red precipitation of nickel dimethylglyoxime is formed when dimethylglyoxime and nickel cation react.
Ni 2+ + 2C 4 H 8 N 2 O 2 → Ni(C 4 H 7 N 2 O 2 ) 2 ↓
Red Precipitate
As a reagent, it is commonly used in the gravimetric estimation of nickel. Gravimetric estimation consists of (1) precipitating nickel by adding dimethylglyoxime, (2) filtering out the precipitate, and (3) calculating nickel's mass by analyzing the precipitate. A chelating agent facilitates the precipitation of organic functional groups with metal ions in this case. Dimethylglyoxime is the chelating agent in this case.
FeSO 4 + 2NH 4 OH + 2C 4 H 8 N 2 O 2 → Fe(C 4 H 7 N 2 O 2 ) 2 + (NH 4 ) 2 SO 4 + 2H 2 O
The presence of red chelate is evident when the solution's pH falls within 5-9. This is because four nitrogen atoms donate their electron pairs instead of the oxygen atoms. Ammonia solution is added to prevent the pH from dropping below 5, which can result in the formation of nickel (II) ions and dissolution of Ni(DMG)2. Adding a small excess of reagent will have no significant impact, but an excessive amount could cause it to precipitate or crystallize with the chelate. The appropriate amount of reagent depends on the quantity of ions present. When ferrous sulfate and ammonium hydroxide combine in the presence of dimethylglyoxime, they produce a compound of iron and ammonium sulfate and release water simultaneously.
Also Read : Nucleophile Formula
Different Applications of Dimethylglyoxime
- In the case of palladium, the precipitation takes the form of a yellow compound after diluting it with hydrochloric acid. It may be used as a precipitant for nickel as well. A bright red voluminous compound precipitates from ammoniacal nickel solution, on the other hand.
- Using DMG, nickel-related dermatitis or similar skin conditions can be detected. As a result, dimethylglyoxime tests can be purchased over the counter in pharmacies in many countries. These tests identify nickel released from watches, jewelry, etc., that come directly into contact with our skin.
- For different metal ions including platinum, palladium and nickel, dimethylglyoxime is used extensively in analytical chemistry as a detection reagent, precipitating reagent, and photometric reagent. Among other things, it contributes to the sustainable recycling of dumped lithium-ion batteries, which are considered hazardous to the environment.
- Dimethylglyoxime is used for precipitating lithium, cobalt, and nickel from waste cathode materials of these batteries.
- Also, DMG has applications in the investigation of iron (III) reduction for determining dissolved iron in seawater. Kinetic experiments are used to study the efficiency of reducing inorganic iron with sulphite under different conditions.
- In seawater, transition metals such as cobalt must be removed with DMG.
Dimethylglyoxime Formula FAQs
Q1. What is the chemical formula of dimethylglyoxime?
Q2. What is the common use of dimethylglyoxime?
Q3. What is the appearance of dimethylglyoxime?
Q4. How is dimethylglyoxime used in nickel testing?
Q5. Are there other uses for dimethylglyoxime?










