
The inorganic compound nitrogen oxide, known as dinitrogen monoxide , has a simple chemical formula. At room temperature, it is colourless and combustible. In this article, we will examine the dinitrogen monoxide formula with examples and its properties and applications. It is also a strong oxidant and is quite comparable to the oxygen molecule.
Concept of Dinitrogen Monoxide
As a powerful oxidizer, it is similar to molecular oxygen at increased temperatures and is colorless and inflammable at room temperature.
In 1772, English chemist Joseph Priestley synthesized this gas. It is famous for its use in aesthetic purposes. However, it has been known to have some adverse health effects. Inhaling it or contacting it with your skin or eyes can be harmful. It is used in rocket propellants and motor racing in order to increase the power output of engines.
It is found to be a main scavenger of stratospheric ozone. 30% of these are due to human activity, mostly agriculture. This gas is typically found in pressurized metal canisters.
It is possible to see these metal canisters lying around outside bars and nightclubs. Ninety-nine percent of their elimination from the body is through the lungs.
Properties of Dinitrogen Monoxide
|
Dinitrogen Monoxide Properties |
|
| Common Name | Dinitrogen Monoxide |
| Other Names | Nitrous Oxide, Dinitrogen oxide, Laughing gas |
| Physical State | Colourless Gas |
| Chemical Formula | N 2 O |
| Melting Point | −88.48 °C |
| Boiling Point | −90.86 °C |
| Density | 1.98 kg/m³ |
| Molar Mass | 44.013 g/mol |
Also Check – Bleaching Powder Formula
Dinitrogen Monoxide Chemical Structure
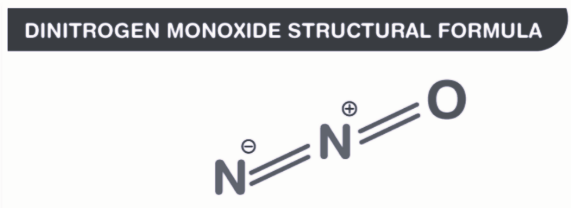
Its chemical formula is;
N 2 O
Its chemical equation is:
2N 2 + O 2 2N 2 O
One of the gases responsible for global warming is dinitrogen monoxide.
Nitrous oxide decomposes violently at high temperatures.
Minor procedures can be anaesthetized using nitrous oxide at modest doses.
In the presence of oxygen, it decomposes at 873 K into oxygen and nitrogen.
Nitrous oxide gas has an oxidation state of +1 for nitrogen.
Also Check – Chemical Bonding Formula
Chemical Properties of Dinitrogen Monoxide
- Nitrous oxide gas has an oxidation state of +1 for nitrogen.
- Sodium azide and ammonia are produced in an exothermic reaction with sodium amide.
2NaNH 2 + N 2 O → NaN 3 + NaOH + NH 3
Nitrogen dioxide is formed when dinitrogen monoxide reacts with oxygen gas.
4N 2 O(g) + 6O 2 (g) → 8NO 2 (g)
Also Check – Aluminium Acetate Formula
Nitrous Oxide Uses
It is sometimes administered before dental treatments to improve relaxation and minimize anxiety as it functions as a moderate sedative and alleviates discomfort. Nitrous oxide gas acts quickly as a sedative, but it has no long-term effects. However, like any other medicine, it may have adverse effects. The Dinitrogen Monoxide Formula is risk-free. Other applications include:
- During dental treatment, reduces unnecessary movement and response.
- Communication and cooperation between the patient are improved.
- Increases the patient's pain tolerance organically.
- Provides mental and physical health care to patients with disabilities.
- Gagging should be reduced.
- The effects of sedatives are amplified.
Safety Measures for Dinitrogen Monoxide
- Keep eyes, skin, and clothing away from contact.
- It is hazardous to use empty containers because they retain product residue.
- Avoid clothing, incompatible materials, and combustible materials.
- Grease and oil should not be allowed to accumulate on reduction valves.
Dinitrogen Monoxide Formula FAQs
Q1. What is the chemical formula for dinitrogen monoxide?
Q2. What is dinitrogen monoxide used for?
Q3. Is dinitrogen monoxide dangerous?
Q4. What are the symptoms of dinitrogen monoxide poisoning?
Q5. What should I do if I think someone has been poisoned by dinitrogen monoxide?










