
Ethanol , also known as ethyl alcohol, can be naturally derived through plant fermentation or by the hydration of ethylene. As an organic compound with the formula C 2 H 5 OH, it serves various purposes such as being added to automotive gasoline, used as a solvent, and synthesized for other organic chemicals. Alkenes like ethene are crucial in understanding its chemical makeup, as they consist of carbon and hydrogen atoms with at least one double bond between two carbons, unlike alkanes where single bonds are present.
Formula of Ethanol
Ethanol is a compound of carbon, oxygen, and hydrogen elements with the formula CH 3 CH 2 OH or C 2 H 5 OH.
Antoine Lavoisier described it, and Nicolas-Theodore de Saussure invented its chemical formula in 1808.
Ethanol's structural formula was published five years later by Archibald Scott Couper.
There are two carbon chains in ethyl alcohol, namely ethane and a hydroxyl group (-OH).
Below are the chemical formula and structural formula of ethyl alcohol (ethanol), but before formulae, it is important to understand hydroxyl groups.
Hydroxyl Group
-OH is a functional group made up of one oxygen atom and one hydrogen atom bonded together. The minus sign indicates that the group is negatively charged.
To name compounds containing this hydroxyl group, the suffix 'ol' is used. This is why ethanol and ethyl alcohol contain the 'ol' suffix.
Ethyl Alcohol Chemical Formula
The chemical formula of ethanol is as follows: 2 carbon atoms, 5 hydrogen atoms, and an OH group.
| Chemical Formula for Ethanol (Ethyl Alcohol) | C 2 H 5 OH |
| The Formula for Ethyl Alcohol | CH 3 CH 2 OH |
The colourless, volatile liquid ethyl alcohol has a pungent odour and is toxic when consumed in large quantities. It is a depressant drug with a flashpoint of 55°F. Ethanol molecules are composed of two carbon atoms, six hydrogen atoms, and one oxygen atom per molecule, but their molecular formula does not provide any structural information. In chemistry, structural information refers to how atoms are connected and how molecules fill space.
Ethyl Alcohol Structural Formula
It is the second most simple alcohol and is represented as:
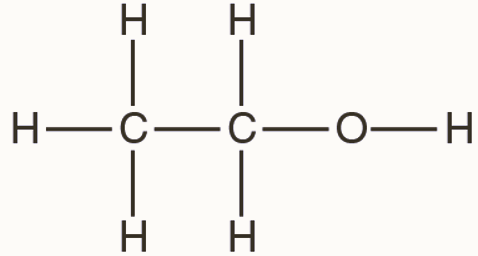
The carbon atoms in an ethanol molecule are sp3 hybridized, that is, they have a free rotation.
The most common use of ethanol is as an antiseptic in the pharmaceutical industry. However, ethanol may also be used in cosmetics and biotechnology.
Also Check – Aluminium Nitrate Formula
Natural Occurrence of Ethanol
Ethanol is a natural byproduct of yeast metabolism, present in all yeast environments and even in overripe fruits. This symbiotic yeast also produces ethanol in the flowers of the Bertam palm and during the germination of various plants. In addition, it can also be found in outer space, where it forms a frozen layer around dust particles in interstellar clouds. Interestingly, trace amounts of ethanol and acetaldehyde have also been observed in the breath of healthy individuals.
Properties of Ethanol
Ethanol remains in its liquid state at room temperature, with a boiling point of 351K and melting point of 156K. As a highly versatile ingredient, it is a key component in all alcoholic beverages, as well as in the production of medicines such as tonics, cough syrup, and iodine tinctures thanks to its solvent properties. Notably, ethanol is completely soluble in water regardless of the amount. However, even small amounts of pure ethanol consumption can be detrimental to one's health. Long-term alcohol consumption can result in various diseases and negative effects on overall health. Oxidizing ethanol with monatomic oxygen yields ethanoic acid, while also being a flammable substance. When burned with oxygen, it produces heat, water, carbon dioxide, and light.
Also Check – Mole Fraction Formula
Solvent Properties
The fact that ethanol is a versatile solvent suggests that it is miscible with water.
- Additionally, it is miscible with acetic acid, benzene, acetone, chloroform, carbon tetrachloride, and diethyl ether, as well as glycerol, ethylene glycol, pyridine, nitromethane, and toluene.
- In preparation of tinctures of iodine, cough syrup, etc., ethanol is used as a solvent.
- Also miscible with ethanol are light aliphatic hydrocarbons like hexane and pentane, as well as aliphatic chlorides like tetrachloroethylene and trichloroethane.
A chain of five or more carbon atoms, whose water solubility decreases rapidly with increasing carbon number, contrasts with the miscibility of ethanol with water. At a certain temperature, mixing dodecane with higher alkanes shows a miscibility gap. The temperature of dodecane is about 13oC. With higher alkanes, the mismatch gap becomes wider and wider. And the temperature for the whole miscibility rises with it.
Also Read – Aluminum Acetate Formula
Uses of Ethanol
- Alcoholic beverages, solvents, scents, flavourings, colourings, medicines, chemical synthesis, and thermometers use ethanol.
- Fuel, solvent, and raw material for making other chemicals, ethanol is a valuable resource.
- Methanol, which is even more toxic than pure ethanol, is sometimes added to it to discourage people from drinking it.
- Camping stoves use methylated spirits, a mixture of ethanol and methanol.
- As a raw material for making esters, which are used as solvents in food flavorings and cosmetics.
- An oxidizing bacteria called acetobacter produces vinegar, a weak solution of ethanoic acid, from ethanol.
Ethanol Formula FAQs
Q1. Is ethanol the same as drinking alcohol?
Q2. Can ethanol be used as a fuel?
Q3. What are the disinfectant properties of ethanol?
Q4. Can ethanol be used for preserving biological specimens?
Q5. Is ethanol toxic if ingested in large quantities?










