
The weak acid formed by chlorine dissolving in water is Hypochlorous Acid . The chemical formula of hypochlorous acid is HOCl, but its molecular formula is HClO. It is referred to as chloranol, chloric acid, and chlorine hydroxide. A simple molecule with an oxygen atom connected to hydrogen and chlorine through single bonds. In the human body, the immune cells produce hypochlorous acid to fight infections.
Hypochlorous Acid Properties
| Hypochlorous Acid Properties | |
| Name | Hypochlorous Acid |
| Alias | Chloric acid, chloranol, chlorine hydroxide and hypochlorite |
| Appearance | Colourless aqueous solution |
| Molecular Formula | HClO |
| Solubility in Water | Soluble |
| Molar Mass | 52.460 g/mol |
Hypochlorous Acid Formula and Structure
Molecularly, it is known as HClO. Its chemical formula is HOCl. Additionally, it has a molar mass of 52.46 g.mol. In addition, it's a simple molecule with oxygen at its center connected to chlorine and hydrogen by a single bond. As shown in the image below, its chemical structure can be written using the common representation:
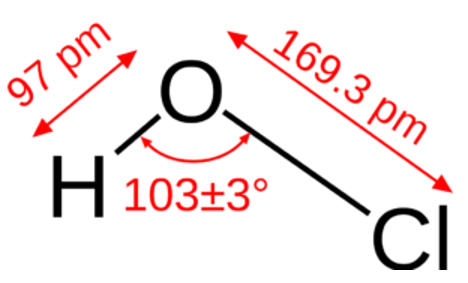
The Occurrence of Hypochlorous Acid
Humans produce them to fight infections and act against a wide range of microorganisms.
Also Check – Calcium Bromide Formula
Preparation of Hypochlorous Acid
In water, chlorine produces hypochlorous acid and hydrochloric acid (HCl):
Cl 2 + H 2 O → HOCl + HCl
Although this reaction is in equilibrium, isolating HOCl from the mixture is challenging. We can obtain stable hypochlorous salt by dissolving chlorine gas in sodium hydroxide or other aqueous essential solutions. Dichlorine monoxide can also be prepared by dissolving it in water.
Cl2 + H 2 O → 2HOCl
Also Check – Ascorbic Acid Formula
Physical Properties of Hypochlorous Acid
As well as being an aqueous solution, it is also colorless, and it is difficult to tell its exact physical properties, as they depend on the solution's concentration. As the molecule is in equilibrium with its anhydride, it cannot be prepared dry or anhydrous.
Chemical Properties of Hypochlorous Acid
A weak acid, it dissociates into hypochlorite ions and hydrogen in aqueous solutions, forming explosive mixtures. As a strong oxidizer, it can form explosive mixtures. Aside from that, it reacts with bases to produce hypochlorite salts. When hypochlorous acid reacts with sodium hydroxide to form sodium hypochlorite (NaOCl), the active ingredient in bleach, sodium hypochlorite is formed.
Also Read: Balancing Chemical Equation Formula
Reactions
Reaction with the Protein Amino Groups
According to Thomas et al., organic chloramines undergo internal rearrangement as they decay, promoting attack on the peptide bond and leading to protein cleavage. Similarly, Davies and McKenna observed that a 10 mM or higher concentration of HClO is necessary to fragment Vivo's proteins. These findings were further supported by later studies, which suggested that chloramine induces a molecular rearrangement, resulting in the release of ammonia and HCl and the formation of an aldehyde. This aldehyde can then react with another amino group to form a Schiff's base, ultimately causing cross-linking and aggregation of proteins. Additionally, this reaction may also occur with lipids.
Reaction with the Lipids
The hypochlorous acid reacts with the unsaturated bonds in lipids while leaving the saturated bonds untouched. This reaction occurs through hydrolysis, where chlorine is added to one carbon and a hydroxyl group to another, forming chlorohydrin. The polar nature of chlorine can disrupt lipid bilayers, potentially increasing their permeability. When this happens in red blood cells, their permeability can also increase. Adding preformed chlorohydrin to red blood cells has a similar effect on permeability. Although cholesterol chlorohydrin has been observed, it does not affect permeability and it is believed that Cl2 is solely responsible for this reaction.
Uses of Hypochlorous Acid
The compound is an excellent oxidizer as well as an effective sanitizer. It is used to make sodium hypochlorite (NaOCl) and calcium hypochlorite (Ca(OCl)2), which are then used to make bleaches, disinfectants, and deodorants. It is also an active sanitizer we use in swimming pools, a wound disinfectant, and a skin cleansing agent in cosmetics.
Hypochlorous Acid Formula FAQs
Q1. What is the chemical formula for hypochlorous acid?
Q2. What is the pH of hypochlorous acid?
Q3. What is the role of hypochlorous acid in disinfection?
Q4. Is hypochlorous acid safe for human use?
Q5. How is hypochlorous acid generated for practical applications?










