Lead II Chloride Formula is expressed as PbCl2, and it is odourless and colourless. Lead (ll) chloride is a white solid compound found in nature as the mineral cotunnite.This lead-based reagent is especially important due to its stability - unlike CCl4 or SiCl4, PbCl4 decomposes at room temperature into the much more stable PbCl2. As elements of group 4 have two oxidation states (+4 and +2), the stability of +4 decreases while +2 increases down the group; this makes PbCl2 much more stable than lead tetrachloride.
What is Lead (II) Chloride Formula ?
Lead (ll) chloride a major reagent containing lead, lead II chloride has a low solubility in water. It is found as the mineral cotunnite in nature. Lead II chloride is the main precursor to numerous organometallic derivatives of lead, like plumbocenes. When molten sodium nitrite reacts with lead II chloride, lead II oxide is formed.Structure of Lead (ll) chloride
By IUPAC, Lead (II) Chloride Formula is also called lead (ll) chloride. There is an isotopic bond distance of 2.44 between each chlorine atom and the central lead atom, and there is 98 degrees of covalent bonding connecting the chlorine atoms to the central lead atom. Understanding the nature of the bond requires analyzing molecules and atoms in their arrangement.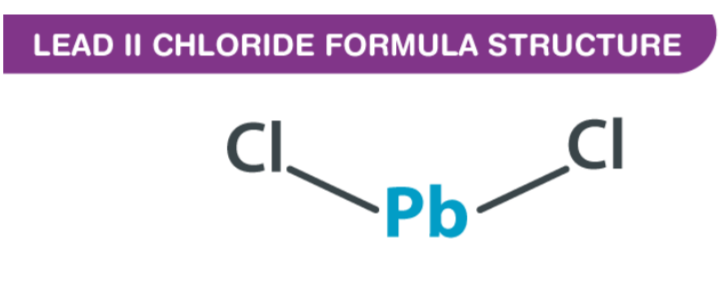 As a result of its atomic number of 82, lead has two electrons that can be donated or shared. Lead has a configuration of Pb 6s2 4f14 5d10 6p2. In contrast, chlorine has an atomic number of 17, which indicates that its electronic configuration is 1s2 2s2 2p6 3s2 3p5, indicating that the shell is unstable due to an unpaired electron in the third orbital.
As a result of its atomic number of 82, lead has two electrons that can be donated or shared. Lead has a configuration of Pb 6s2 4f14 5d10 6p2. In contrast, chlorine has an atomic number of 17, which indicates that its electronic configuration is 1s2 2s2 2p6 3s2 3p5, indicating that the shell is unstable due to an unpaired electron in the third orbital.
Also Read : Surface Chemistry Formula
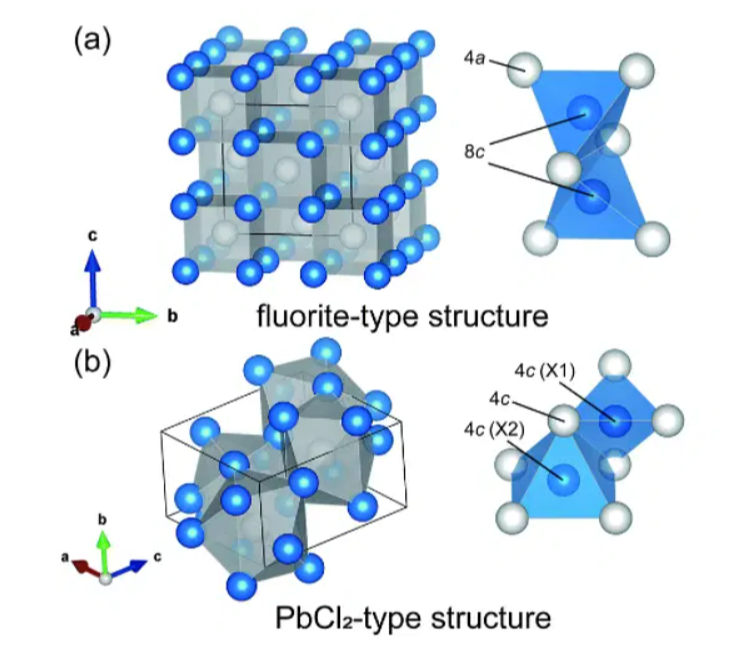 An octane molecule is formed by sharing each of the two pair electrons of lead present in the 6s shell with two chlorine atoms, resulting in a stable molecule. Due to its twisted structure, Lead (II) Chloride Formula molecules have the following structural formula.
As a result, le's structural formula Each lead atom in lead (ll) chloride is surrounded by nine chloride ions, which resembles a tricapped triangular prism molecular geometry in which nine atoms or groups of atoms or ligands are arranged around the central atom in a triangle, each atom attached to one of its three faces.
An octane molecule is formed by sharing each of the two pair electrons of lead present in the 6s shell with two chlorine atoms, resulting in a stable molecule. Due to its twisted structure, Lead (II) Chloride Formula molecules have the following structural formula.
As a result, le's structural formula Each lead atom in lead (ll) chloride is surrounded by nine chloride ions, which resembles a tricapped triangular prism molecular geometry in which nine atoms or groups of atoms or ligands are arranged around the central atom in a triangle, each atom attached to one of its three faces.
Also Check - Malic Acid formula
There are six chloride ions in each vertice of the triangle prism with lead as its center atom, and three beyond the surfaces of each rectangular prism face. As a result, the chloride ions are not evenly spaced. Seven of the nine chlorine atoms are located between 280 and 309 pm, while the other two are between 270 and 280 pm. Therefore, PbCl2 forms an orthorhombic needle structure, also called an orthorhombic dipyramidal, which has a point group of 2m / 2m / 2m. Each lead atom has a coordination number of 9. As a result, the structural formula of lead chloride can be found below in terms of its coordinate geometry. Ad chloride molecules are shown in the following diagram.Also Check - Iron (III) Hydroxide Formula
Properties of Lead (ll) chloride
| Property Name | Value |
| Molar mass | 278.10 g / mol |
| The chemical formula of lead chloride | PbCl2 |
| Appearance | White and odorless solid |
| Hardness | 1.5 - 2mohs scale |
| Density | 5.85 g /cm3 |
| Boiling point | 950 oC or 1740 oF or 1220 K |
| Melting point | 501 oC or 934 oF or 774 K |
| Crystal structure | orthorhombic, oP12 |
| Space group | Pnma No. 62 |
| Solubility in water | 0.99 g in 1L of water at 20 oC |
| Solubility product (Ksp) | 1.7 x 10-5 at 20 oC |
| Solubility | It is slightly soluble in HCl and ammonia, insoluble in alcohol, soluble in hot water, in the presence of alkali hydroxide, and in concentrated HCl. (>6M) |
| Magnetic susceptibility | -73.8 x 10-6 cm3 / mol |
| Refractive index | 2.199 |
| Std. molar entropy | 135.98 J K-1 mol-1 |
| Std. enthalpy of formation | -359.4 KJ / mol |
Also Check - Combined Gas Law Formula
Synthesis of Lead II Chloride
The twofold displacement reaction, or metathesis reaction, occurs when lead (ll) compounds react with an aqueous solution of chloride compounds such as HCl, NaCl, or KCl. With Soluble Lead Compound Lead (ll) Nitrate : Pb(NO3)2 + 2NaCl (aq) → PbCl2 (s) + NaNO3 (aq) Lead (ll) Acetate : Pb(CH3COO)2 (aq) + HCl (aq) → PbCl2 (s) + 2CH3COOH With Insoluble Lead Compound Lead (ll) Carbonate : PbCO3 + 2 HCl (aq) → PbCl2 (s) + CO2 (g) + H2O (aq) Lead (ll) Dioxide : PbO2 (s) + 4 HCl (aq) → PbCl2 (s) + Cl2 (g) + 2 H2O (aq) Lead (ll) Oxide : PbO (s) + 2 HCl (aq) → PbCl2 (s) + H2O (aq) Lead (ll) chloride is formed when copper (ll) chloride is reduced in the presence of lead metal. The reaction is as follows: Pb (s) + CuCl2 → PbCl2 (s) + Cu (s) As a result of direct chlorination of lead metal by chlorine gas, lead (ll) chloride is also produced. The reaction is as follows: Pb (s) + Cl2 (g) → PbCl2 (s)Also Check - Ionic Compound Formula
Uses of Lead II Chloride
- The manufacture of infrared transmitting glass involves the use of lead II chloride.
- According to specialists, the iridescent surface of Aurene glass is achieved by spraying it with lead II chloride and warming it in regulated conditions. This chemical is also used in the manufacture of aurene glass, an attractive glass.
- By using lead II chloride as an intermediary, bismuth (Bi) ore can be refined.
- To remove traces of acidic elements, the ore containing Bi, Pb, and Zn is first treated with molten caustic soda.
- Silver and gold are then removed by using the Parkes desilverization process.
- In the ore, Bi, Pb, and Zn are currently present. Experts use Cl2 gas at 500°C to treat it. First, ZnCl2 forms, then it is removed.
- Next, PbCl2 emerges and is eliminated, leaving only pure Bi. Finally, BiCl3 would form.
Lead (II) Chloride Formula FAQs
What is the Lead (II) Chloride Formula ?
Is Lead (II) Chloride soluble in water?
What is the common name for Lead (II) Chloride?
Is Lead (II) Chloride toxic?
What is the color of Lead (II) Chloride?