
Butan-1-Ol , also known as 1-Butanol or n-butyl alcohol, is a primary alcohol with the chemical formula C 4 H 9 OH and a four-carbon structure. It is naturally occurring as a minor byproduct of sugar and carbohydrate fermentation and can be found in various foods and beverages. Its isomers include isobutanol, butan-2-ol, and tert-butanol. This colorless and refractive liquid has a harsh, mildly alcoholic aroma reminiscent of bananas. Small amounts are also produced in the human body by gut microbes. It has many uses such as organic chemical synthesis, plasticizers, and detergents.
Structure of Butan-1-Ol
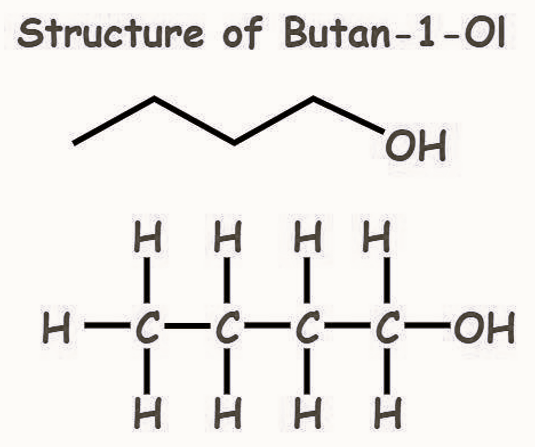
The chemical formula of butan-1-ol is C 4 H 10 O (or) C 4 H 9 OH, and its expanded form is CH 3 (CH 2 ) 3 OH (or) CH 3 CH 2 CH 2 CH 2 OH.
It consists of four main isomers, which are produced by changing the position of the OH group or the positions of carbon atoms that are joined together. Isobutanol, butan-2-ol, and tert-butanol are the isomers of 1-butan-ol. 2-Butan-2-ol is a straight-chain isomer with the OH group at an internal carbon, isobutanol is a branched isomer with the OH group at a terminal carbon, while tert-butanol is a branched isomer with an internal carbon.
Preparation of Butan-1-Ol
However, butanol can also be produced by fermenting glycerol, mannite, starches, and sugars in general by bacteria such as Bacillus butylicus. Crotonaldehyde, formed from acetaldehyde, was previously used to produce butanol. Alternatively, industrial fermentation can be used to produce it with Clostridium acetobutylicum.
In this process, n-butyraldehyde is formed by oxo-reacting propylene with n-butyraldehyde. This produces a mixture of n-butyraldehyde and isobutyraldehyde. In order to separate the individual isomers of the aldehyde from this mixture, either it can be separated first and hydrogenated individually, or it can be hydrogenated directly and the resultant mixture of n- and isobutyl alcohol is then separated by distillation.
Also Check – Aluminium Nitrate Formula
Properties of Butan-1-Ol
| Chemical formula | C 4 H 10 O (or) C 4 H 9 OH |
| IUPAC name | Butan-1-Ol |
| Molecular weight/Molar mass | 74.123 g/mol |
| Appearance | Colourless, refractive liquid |
| Odor | harsh, mildly alcoholic, sweet, and banana-like odour |
| Boiling point | 117.7 °C |
| Melting point | −89.8 °C |
| Density | 0.81 g/cm3 |
| Solubility | Easily soluble in acetone and miscible with ethanol, ethyl ether |
| Acidity (pKa) | 16.10 |
| Refractive index | 1.3993 (20 °C) |
| Dipole moment | 1.66 D |
Uses of Butan-1-Ol
- Butan-1-ol has various uses in the manufacturing industry.
- It serves as a solvent and chemical intermediate and an artificial flavouring agent for baked goods, candies, ice cream, and other products. Furthermore, it is utilized as a solvent in a range of applications including surface coatings, paints, lacquers, and resins.
- Butanol has also been proposed as a potential substitute for diesel fuel and gasoline.
- Its versatility makes it a common ingredient in household and commercial products such as arts & crafts supplies, cleaning products, metal polish, and pesticides.
Also read - Surface Chemistry Formula
Exposure
People who work in industries that use n-butyl alcohol will likely be exposed to it. A person may be exposed to n-butyl alcohol by eating foods containing it or by breathing in fumes from cooking certain foods. These include varnishing automobiles, painting shops, and fabric coatings.
It is also found in surface water and air. It is particularly prevalent in new construction's indoor air. It breaks down in the air when it reacts with radicals.
Microorganisms break down n-butyl alcohol in soil or water, but it does not accumulate in aquatic organisms.
Also Check – Bond Order Formula
Risk Factors
The liquid or vapor form of n-butyl alcohol may irritate the eyes. As well as blurring vision, increasing tear production, light sensitivity, and burning, n-butyl alcohol can also cause irritation to the skin and upper respiratory tract. High doses of n-butyl alcohol can cause depression of the central nervous system.
Butan 1 ol Formula FAQs
Q1. What is the chemical formula of 1-butanol?
Q2. Is 1-butanol a primary alcohol?
Q3. What are the common uses of 1-butanol?
Q4. Is 1-butanol toxic to humans?
Q5. What are the different isomers of butanol, and how do they differ?










