
Retention Factor Formula: The Retention Factor formula constitutes a fundamental calculation in chromatography, a technique used to separate different components within mixtures. In paper chromatography, a solution is applied at the base level, allowing the solute and solvent to traverse a certain distance. The solution's constituents are retained on the paper, and the extent of this retention is quantified using the Retention Factor.
Definition of Chromatography
Chromatography serves as the method used to divide different substances into their distinct components. Several chromatographic techniques are utilized for this purpose, such as:
- Thin Layer Chromatography (TLC)
- High-Performance Thin-Layer Chromatography (HPTLC)
- High-Performance Liquid Chromatography (HPLC)
- Gas Chromatography (GC)
Chromatography involves two fundamental principles for its execution:
Partition Chromatography
Adsorption Chromatography
Next, we will discuss in a detailed understanding of each:
Partition Chromatography: This method involves the separation of compounds based on the interaction between stationary and mobile phases within the components.
Adsorption Chromatography: This technique separates compounds by relying on the selective adsorption of mixture components.
Definition of Retention Factor
The Retention Factor, also known as the R f value, represents the ratio between the distance covered by the solute and the distance covered by the solvent within a chromatographic system. Being a unitless quantity due to its nature as a ratio of similar distances, the R f value is a significant metric in chromatography. Its calculation involves the formula:
Retention factor (R f ) = Distance traveled by the solute / Distance traveled by the solvent
The R f value of a compound is contingent upon the solute's inclination towards the stationary and mobile phases. If a substance component shows a strong affinity toward the stationary phase, its movement is slower, while a component with lower affinity to the stationary phase moves faster.
Retention Factor Formula
The Retention Factor, symbolized as Rf, is determined as the ratio between the distance covered by the solute and the distance covered by the solvent. The mathematical representation is expressed as:
Retention Factor Formula (R f ) = (Distance Traveled by Solute) / (Distance Traveled by Solvent)
The Retention Factors of diverse solutions serve as a means to compare their behaviors under various combinations of solutes and solvents. R f values differ across different solutions, influenced by interactions among individual components and the concentration of those components in the sample, ultimately impacting the Retention Factor value.
Determining the Retention Factor (R f value) of a Component
The R f value, or retention factor, of a component is straightforwardly computed using the Retention Factor Formula:
Retention Factor (R f ) = Distance traveled by the solute / Distance traveled by the solvent
An example illustrating this process is detailed in the included image for better comprehension.
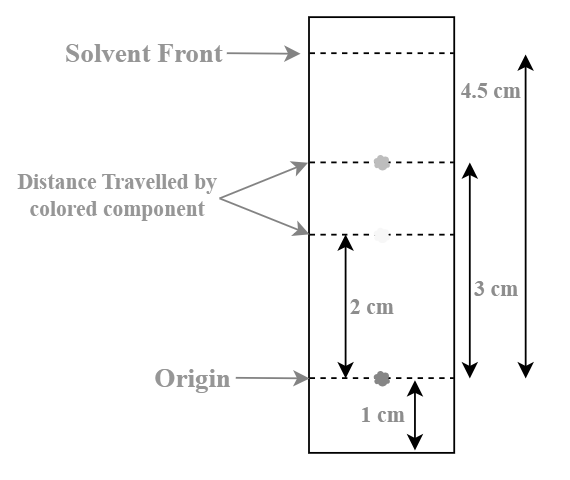
From the illustration provided:
A mixture is placed on the TLC plate (depicted as the red spot) at the origin point, coated with silica gel serving as the stationary phase.
The solvent front, marked at the 6 cm mark, allows the mobile phase to flow from the origin.
The image displays two distinct solutes in the mixture, one yellow and the other orange.
In this scenario, the distance covered by the yellow spot is 2 cm, and the distance covered by the orange spot is 3 cm.
Hence, the R f values for the orange and yellow spots can be easily calculated as follows:
R f of yellow spot = 2/4.5 ≈ 0.444
R f of orange spot = 3/4.5 ≈ 0.667
Factors Affecting Retention Factor (R f value)
Several factors impact the retention factor (Rf value) of the solute or solvent within a mixture, including:
Thickness of the stationary phase layer
Moisture content on the TLC plate
Characteristics of the TLC plate
Saturation level of the mobile phase
Volume of the mobile phase
Environmental temperature
Quantity of the sample utilized
Retention Factor Formula Solved Example
Example 1: Determine the retention factor when the solute and solvent travel distances of 5cm and 10cm respectively, from the baseline situated at 0cm.
Solution:
Provided:
Distance covered by Solute = 5cm
Distance covered by Solvent = 10cm
Retention Factor Formula (R f ) = (Distance Traveled by Solute Particle) / (Distance Traveled by Solvent Particle)
R f = 5/10
R f = 1/2 = 0.5
Hence, the calculated retention factor for the given solution is 0.5.
Explore Now Online Course of Class 9 Neev Fastrack 2024 and Class 10 Udaan Fastrack 2024 to enhance your Maths knowledge. and build a strong foundation.
| Related Links | |
| U Substitution Formula | Vertex Formula |
| Foil Formula | Function Notation Formula |
Retention Factor Formula FAQs
What is the Retention Factor (Rf) in chromatography?
How is Rf calculated?
What does the Rf value indicate?
Why is Rf significant in chromatography?
What Affected the Rf value?










