
Electrochemistry cells consist of two half-cells where oxidation and reduction reactions take place. It consists of two electrodes made of metals with different reactivity and due to the difference in electrode potential chemical reactions take place resulting in electrical energy.
Electrochemistry Cell
Overall cell reaction: Oxidation half-reaction + Reduction half-reaction gives overall cell reaction.
Representation:
Zn | Zn 2+ (1 M) ∣ ∣ Cu 2+ (1 M) ∣ Cu
Measurement of Electrode Potential
Standard electrode potential is measured using the Standard Hydrogen Electrode (SHE) as a reference.
Cell Potential ( E cell ) = Cathode Potential (E cathode ) - Anode Potential (E anode )
Nernst Equation
For the reaction:
aA + bB→ cC + dD
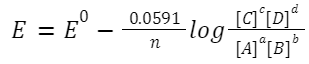
Where n is the number of electrons transferred. Equilibrium Constant from Nernst Equation At equilibrium, E=0, so:
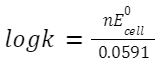
where K is the equilibrium constant.
Electrochemical Cell and Gibbs Energy of the Reaction:
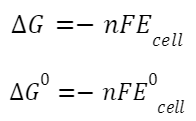
where ΔG is the change in Gibbs free energy,
F is Faraday's constant, and
E is the cell potential.
![]()
k- equilibrium constant
Conductance of Electrolytic Solutions
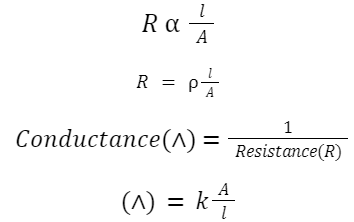
where κ is conductivity,
A is the area, and
l is the length of the solution between electrodes.
K = 1/ρ
where ρ is the resistivity.
Specific conductance: k = 1/R × l/A
where l is the distance between the electrodes,
R is resistance, and
A is area.
Measurement of the Conductivity of Ionic Solutions:
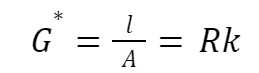
Change in Conductivity and Molar Conductivity with Concentration
Molar conductivity:
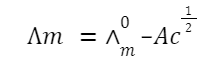
where C is the concentration.
Also Check – Atomic Mass Formula
Kohlrausch’s law
For strong electrolytes:
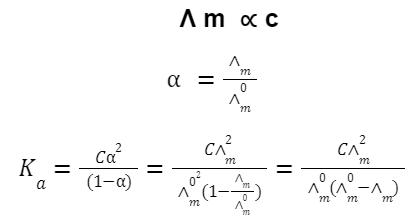
For weak electrolytes:
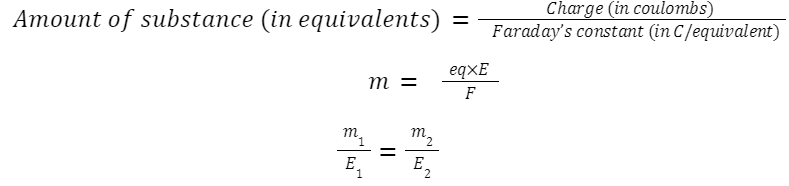
Also Check – Tungstic Acid Formula
Faraday’s Law of Electrolysis
First law:
Mass of substance deposited ∝ ∝ amount of electricity passed.
Charge: Q=It
Second law: img
when the same amount of electricity is passed through different electrolytic cells.
where m is the mass, E is the equivalent weight, m = eq×E/F and F is Faraday's constant.
Batteries
Primary Batteries (Non-rechargeable):
Example: Alkaline battery
Secondary Batteries (Rechargeable):
Example: Lithium-ion battery
EMF: cathode − anode E = E cathode −E anode
Examples:
Lead-Acid Battery:
Anode (Discharge): Pb+SO 4 2- →PbSO 4 +2e –
Cathode (Discharge): PbO 2 + 4H + + SO 4 2- + 2e − → PbSO 4 + 2H 2 O
Overall (Discharge): Pb + PbO 2 + 2H 2 SO 4 → 2 PbSO 4 + 2H 2 O
Nickel-Cadmium (Ni-Cd) Battery:
Anode (Discharge): Cd+2OH − →Cd(OH) 2 +2e –
Cathode (Discharge): NiO(OH) +H 2 O + e − →Ni(OH) 2 + OH –
Overall (Discharge): Cd+ 2NiO(OH)+ 2H 2 O→Cd(OH) 2 +2Ni(OH) 2
Lithium-ion Battery: (Reactions can vary depending on the materials used; here's an example using LiCoO 2 and graphite)
Anode (Discharge): LiC 6 →C 6 +Li + +e –
Cathode (Discharge): LiCoO 2 +Li + +e − →Li 2 CoO 2
Overall (Discharge):
LiCoO 2 + LiC 6 → 2Li 2 CoO 2
Also Check – Bond Order Formula
Corrosion
An example of a corrosion reaction is:
4 Fe + 3O 2 + 6H 2 O → 4 Fe(OH) 3
Anode: Fe→Fe 2+ +2e −
Cathode: O 2 + H 2 O + 2e − → 2OH −
Fuel Cells
Fuel cells convert the chemical energy from a fuel into electricity through an electrochemical process. The most common type is the hydrogen-oxygen fuel cell: Hydrogen-Oxygen Proton Exchange Membrane (PEM) Fuel Cell:
Anode (Oxidation): 2H 2 →4H + + 4e –
Cathode (Reduction): O 2 + 4H + + 4e − → 2H 2 O
Overall Reaction: 2H 2 + O 2 →2H 2 O
Electrochemistry Formula FAQs
Q1. What does a salt bridge do?
Q2. What is the significance of a positive cell potential?
Q3. What is an equilibrium potential?
Q4.What is overpotential?
Q5. Why do batteries die?










