
Graham's Law Formula: To grasp Graham's law of diffusion, it is essential to first acquaint ourselves with the concepts of diffusion and its rate. While diffusion and effusion are often used interchangeably, they hold distinct definitions. Diffusion pertains to the process by which particles from one gas disperse into another, introducing a substantial degree of disorder into the entire system. Moreover, the phenomenon of diffusion can also be observed at slower rates within liquids and solids.
This behavior is primarily influenced by variations in concentration levels, where particles tend to migrate from regions of low concentration to those of higher concentration. An everyday illustration of this process occurs when we apply perfume or scented spray in one corner of a room, subsequently detecting the scent permeating throughout the entire space, thereby illustrating the principle of diffusion.
The transformation in the distribution of diffusing molecules as time progresses is termed the rate of diffusion. The rate of diffusion for a gas is inversely proportional to the square root of its volume or density. This relationship is encapsulated in the rate of diffusion formula:
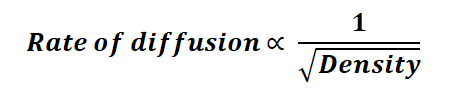
Or
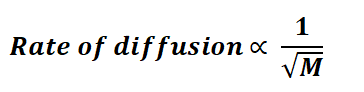
Gas's molar mass is represented as M.
Graham's Law of Effusion Formula
When gaseous particles move through a small opening into a vacuum, open space, or any other gas or atmosphere, this phenomenon is referred to as effusion. During effusion, the molecules of a substance attempt to exit a sealed container through a small opening. A prime example of effusion can be observed in balloons. When a hole is made in a balloon, the gas inside begins to escape into the surrounding atmosphere, causing the balloon to deflate. This process can be described as the effusion of gas into the atmosphere.
The rate at which particles escape from a confined space over time is termed the rate of effusion. Now, let's delve into the formula for the rate of effusion. Since effusion is inversely related to both density and the molar mass of the gas, we can express the equation as follows:
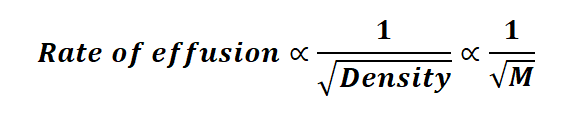
Graham's law of diffusion marked a significant milestone in the field of chemistry. This law, also known as Graham's law of diffusion, was discovered by Thomas Graham in 1848. Through his investigations into the rate of effusion, he unveiled a fundamental principle: gases with heavier molecules move more slowly than those with lighter particles. Graham's law of effusion or diffusion postulates that, under conditions of constant temperature and pressure, atoms with higher molar mass effuse at a slower rate than atoms with lower molar mass. Additionally, Graham provided an equation to quantify the rate at which molecules escape, namely the rate of diffusion.
Furthermore, this law asserts that the square root of the molar mass is inversely proportional to the rate of effusion. This principle gives rise to the Graham's law of diffusion formula. This formula enables us to compare the rates of effusion for two gases under conditions of constant temperature and pressure. If we assume that r1 and r2 represent the rates of effusion for two gases, and M1 and M2 represent their respective molar masses, the formula can be expressed as follows:
r1/r2 = M2/M1
Or
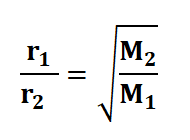
M1 represents the molar mass of the first gas,
M2 designates the molar mass of the second gas,
Rate1 denotes the rate of effusion of the first gas,
Rate2 signifies the rate of effusion for the second gas.
This relationship asserts that the rate of diffusion or effusion is inversely correlated with its molecular mass.
Graham's Law Formula Solved Example
Example 1: Calculate the molar mass of an unknown gas when its diffusion rate is four times that of hydrogen (H2), and the molar mass of hydrogen is approximately 2 g/mol.
Solution:
Given that the diffusion rate is four times that of hydrogen, we establish the ratio of diffusion rates as 4/1, which can be expressed as:
r1/r2 = 4/1
Since the molar mass of hydrogen (H2) is known to be approximately 2 g/mol, we can apply Graham's law:
r1 * r2 = M2 / M1
Substituting the values:
4 * 1 = M2 / 2
Solving for M2:
M2 = 8 g/mol
Hence, the molar mass of the unknown gas (M2) is 8 g/mol.
| Related Links | |
| Hydrogen gas Formula | Hydrofluoric Acid Formula |
| Hydrochloric Acid Formula | Hydrazine Formula |
Graham's Law Formula FAQs
What is Graham's Law of Diffusion?
How does Graham's Law of Diffusion relate to gas particles?
Why is Graham's Law of Diffusion important?
What does the rate of diffusion tell us about a gas?










