
The electronic configuration of copper (Cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This unique configuration is characterized by one electron in the 4s orbital and ten electrons in the 3d orbital, which differs from the typical filling order. Copper's 3d¹⁰ configuration in the third energy level (shell) is attributed to the increased stability of a half-filled or fully-filled d subshell. This electronic configuration of copper is responsible for its exceptional electrical conductivity and has made it a valuable material in various industrial applications, including electrical wiring and electronics. The electronic configuration of copper is often a topic of interest due to its peculiar arrangement of electrons.
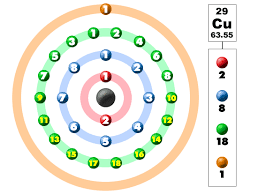
Electronic Configuration of Copper in Shells
Copper's electronic configuration is usually represented as 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This configuration highlights the distribution of electrons in different energy levels, or shells. The 1s², 2s², and 2p⁶ represent the first and second shells, while the 3s² and 3p⁶ denote the third shell. The 4s¹ and 3d¹⁰, which is unusual, belong to the fourth shell. Copper's anomalous electronic configuration in the 4s and 3d orbitals is due to the energy levels of these orbitals.
Also Read: Lead Acetate Formula
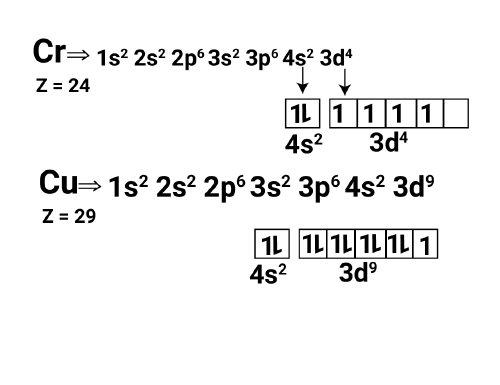
Electronic Configuration of Copper and Chromium
Both copper and chromium exhibit unique electronic configurations in their respective elements. Chromium, with an atomic number of 24, has the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵. Similarly, copper, with an atomic number of 29, has the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. These elements exhibit exceptions to the expected electron filling pattern due to the increased stability achieved by having half-filled or fully-filled d orbitals.
Electronic Configuration of Copper in KLMN Shell
Copper's electrons are distributed across various shells, with the KLMN shell containing the most significant number of electrons. In this context, K represents the first shell, L the second shell, M the third shell, and N the fourth shell. Copper's electronic configuration in these shells can be summarized as follows:
- K shell (1s²)
- L shell (2s² 2p⁶)
- M shell (3s² 3p⁶ 4s¹ 3d¹⁰)
- N shell (empty)
Also Read: Magnesium Iodide formula
Electronic Configuration of Copper 29
The atomic number of copper is 29, and its electronic configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This configuration represents the distribution of 29 electrons within the various orbitals of copper's atomic structure.
Electronic Configuration of Copper 2 Plus (Copper Ion)
Its electronic configuration changes when copper loses two electrons to form a +2 ion. In the case of copper(II) ions (Cu²⁺), the electronic configuration becomes 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸. The loss of two electrons results in the 3d orbital becoming half-filled, achieving a more stable electron configuration.
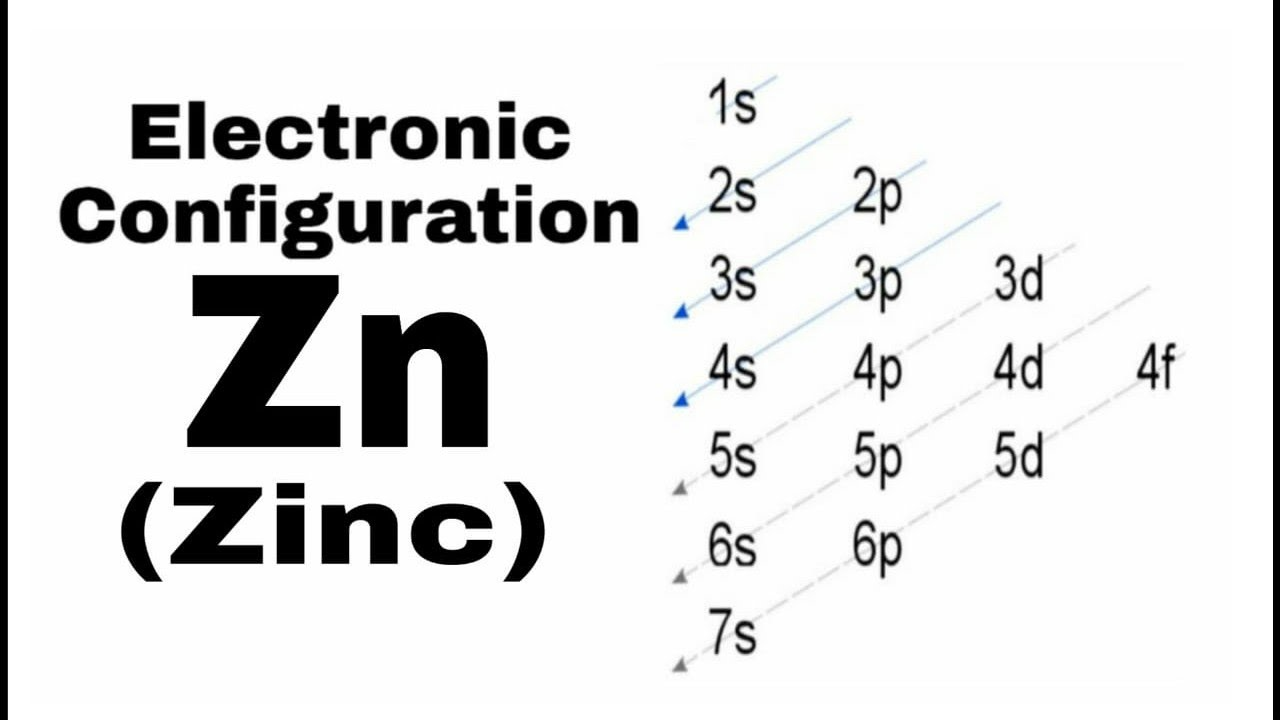
Electronic Configuration of Copper and Zinc
Copper and zinc are neighboring elements in the periodic table and exhibit different electronic configurations. Copper's electronic configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰, while zinc, with an atomic number of 30, has the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰. Zinc has a filled 4s orbital, making it a transition metal that does not exhibit the unique electron distribution found in copper.
The electronic configuration of copper is intriguing due to its unusual arrangement of electrons in the 4s and 3d orbitals. Understanding these electronic configurations is essential for comprehending the chemical properties and reactivity of copper in various chemical reactions.
Also Read: Lead Iodide Formula
Properties of Copper
Copper is an essential metal known for its excellent electrical and thermal conductivity, making it a vital material in electrical applications. Its malleability and corrosion resistance have also contributed to its use in a wide range of industries, from electronics to construction. Additionally, its distinctive reddish-brown color and metallic luster make it a popular choice for decorative and artistic purposes.
| Properties of Copper | |
|---|---|
| Property | Description |
| Atomic Symbol | Cu |
| Atomic Number | 29 |
| Atomic Weight | 63.546 amu |
| Classification | Transition Metal |
| Melting Point | 1,984.32°C (3,604.9°F) |
| Boiling Point | 2,562°C (4,644°F) |
| Density | 8.96 grams per cubic centimeter (g/cm³) |
| Color | Reddish-brown |
| Crystal Structure | Face-centered cubic (FCC) |
| Electrical Conductivity | Excellent electrical conductor |
| Thermal Conductivity | High thermal conductivity |
| Malleability | Highly malleable and ductile |
| Luster | Metallic luster |
| Tensile Strength | High tensile strength |
| Corrosion Resistance | Resistant to corrosion, forming a protective oxide layer |
| Reactivity | Reacts slowly with oxygen and moisture, forming patina |
| Magnetic Properties | Non-magnetic (diamagnetic) |
| Uses | Electrical wiring, plumbing, coins, jewellery, and more |
| Related Links | |
| Electrochemistry | P-Block |
| Chemical Kinetics | D-block |
Electronic Configuration of Copper FAQs
What is the electron configuration of copper 29?
What is the electronic configuration of an element 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹?
How do you write the electron configuration for copper?









