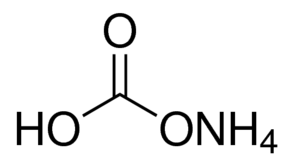
Ammonium Bicarbonate Formula: Ammonium bicarbonate formula is (NH 4 )HCO 3 . It is an inorganic compound. This substance is associated with a multitude of names. From a chemical speaking. It can be defined as the bicarbonate salt originating from the ammonium ion. It presents itself as a colourless solid that undergoes swift decomposition into carbon dioxide, water, and ammonia.
Ammonium Bicarbonate Formula Structure
Ammonium Bicarbonate Formula is (NH 4 )HCO 3 . The chemical composition of ammonium bicarbonate consists of the ammonium cation NH 4 + and the bicarbonate anion HCO 3 – .
Ammonium Bicarbonate Formula Physical Properties
Below are some physical properties of Ammonium Bicarbonate .
Ammonium Bicarbonate Formula is (NH 4 )HCO 3 .
Ammonium bicarbonate is a white crystalline solid.
It has a density of 1.59 grams per milliliter.
It typically remains solid at a temperature of 41.9 degrees Celsius.
It has a robust aroma of ammonia.
Ammonium bicarbonate is highly soluble in water.
It emits an observable pungent smell.
Ammonium Bicarbonate Formula Chemical Properties
The chemical properties of Ammonium Bicarbonate can be summarized as follows:
It easily dissolves in water and forms a mildly alkaline solution.
Ammonium bicarbonate is generally insoluble in many chemical organic solvents..
Ammonium bicarbonate is stable if the temperature is near 25 degree celsius. When temperature rises above the 36 degree celsius. It decomposes into ammonia, carbon dioxide, water, and greenhouse gases. And absorb energy from environments.
NH 4 HCO 3 → NH 3 + CO 2 + H 2 O
ABC reacts with acids to produce carbonic acid gas. It also reacts with bases, resulting in the production of ammonia.
Production of Ammonium Bicarbonate
Ammonium Bicarbonate Formula is (NH 4 )HCO 3 . Ammonium bicarbonate is produced by combining carbon dioxide (CO 2 ) and ammonia (NH 3 ) in the following chemical reaction:
CO 2 + NH 3 + H 2 O → (NH 4 ) HCO 3
This reaction takes place under cold conditions as ammonium bicarbonate is thermally unstable. It is allowing the product to precipitate as a white solid. Approximately 100,000 tons of ammonium bicarbonate were produced using this method in 1997.
When exposed to air. This compound releases ammonia and reverts to its ammonium bicarbonate form. Ammonia gas can convert (NH 4 ) 2 CO 3 and H 2 O into the standard carbonate ((NH 4 ) 2 CO 3 ) when passed into a concentrated solution of the carbonate, which is a mixture of (NH 4 )HCO 3 in a 2:1:1 ratio. The compound can also be obtained in a crystalline state from a solution prepared at around 30 °C.
Uses of Ammonium Bicarbonate
Here are the various commercial and general uses of ammonium bicarbonate:
It serves as a leavening agent in the food industry for products such as crackers, cookies, and cream-puff doughs.
Ammonium bicarbonate is utilized in the production of porous plastics, ceramics, dyes, and pigments.
It finds application in the removal of scale from boilers and functions as a foaming agent in rubber manufacturing.
It is effective in removing gypsum deposits from heat exchangers and processing equipment.
Adding ammonium bicarbonate to compost heaps accelerates decomposition, making it a valuable component in creating fertilizers.
Due to its volatile nature, it is used as a reliable buffer in pharmaceutical processes like lyophilization (freeze-drying) for the production of injectable and oral dosage forms.
It is used in the production of both human and veterinary medications.
In addition to its leavening role, it is used in certain food processing applications and even in cough syrups and as an antacid.
It serves as a fertilizer and pH buffer in chemical laboratories and has applications in the manufacturing of dyes, pharmaceuticals, catalysts, ceramics, plastics, and various other industrial products.
In chemical purification processes, such as high-performance liquid chromatography, ammonium bicarbonate is used to create alkaline buffering solutions, facilitating the recovery of the desired compounds through freeze-drying.
Ammonium Bicarbonate formula is (NH 4 ) 2 CO 3 . It is an inorganic compound with a white crystalline appearance. It has high solubility in water, and a strong ammonia-like odor. It dissolves in water to create an alkaline solution but remains stable at moderate temperatures. It finds diverse applications, from being a leavening agent in the food industry to use in pharmaceuticals, ceramics, and industrial processes. Its versatility makes it a valuable compound in various sectors.
| Related Links | |
| Strontium Sulfate Formula | Strontium Chloride Formula |
| Barium Bromide Formula | Barium Fluoride Formula |
Ammonium Bicarbonate Formula FAQs
What is the formula of Ammonium Bicarbonate?
What is the appearance of Ammonium Bicarbonate?
What is the density of Ammonium Bicarbonate?
At what temperature does Ammonium Bicarbonate melt?
What is the odor of Ammonium Bicarbonate?
Is Ammonium Bicarbonate water-soluble?