
Ammonium bromide formula, a chemical compound with various applications, is an intriguing substance with a well-defined chemical structure. In this article, we will explore the ammonium bromide formula, its structure, molecular composition, equations, and ionic form, and even delve into a closely related compound, cetyl trimethyl ammonium bromide.
Ammonium Bromide Formula
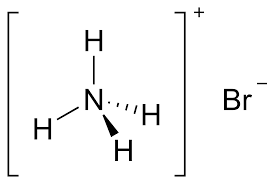
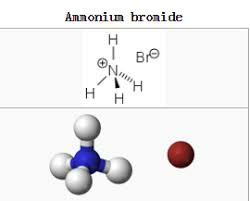
Ammonium bromide is a chemical compound represented by the formula NH₄Br. This formula provides crucial information about the composition of the compound. "NH₄" signifies the ammonium ion (NH₄⁺), which is a positively charged polyatomic ion composed of one nitrogen atom (N) and four hydrogen atoms (H). "Br" represents bromine, a halogen element.
Also Read: Methane formula
Ammonium Bromide Formula Structure
The structure of ammonium bromide formula reflects the arrangement of its constituent atoms. In the case of NH₄Br, the ammonium ion has a tetrahedral structure. The nitrogen atom lies at the center of the tetrahedron, with four hydrogen atoms surrounding it, forming four equal N-H bonds. The bromine atom forms an ionic bond with the ammonium ion. The overall structure can be depicted as follows
Also Read: Octane formula
Ammonium Bromide Formula Molecular
The molecular composition of ammonium bromide formula describes the actual atoms and their quantity within a single molecule of the compound. In this case, a single molecule of ammonium bromide consists of one ammonium ion (NH₄⁺) and one bromide ion (Br⁻) held together by ionic bonds. These ions are electrically attracted to each other, forming a stable compound.
Ammonium Bromide Equation
The ammonium bromide equation can refer to various chemical reactions or processes involving this compound. For example, when ammonium bromide dissolves in water, it dissociates into ammonium (NH₄⁺) and bromide (Br⁻) ions. This can be represented by the equation:
NH₄Br (s) → NH₄⁺ (aq) + Br⁻ (aq)
This equation illustrates the dissociation of solid ammonium bromide into its constituent ions in an aqueous solution.
Ammonium Bromide Ionic Formula
The ionic formula of ammonium bromide is NH₄⁺Br⁻, representing the ions formed when the compound is dissolved in water. The ammonium ion (NH₄⁺) is positively charged, while the bromide ion (Br⁻) is negatively charged. These ions are attracted to each other due to their opposite charges, forming an ionic compound.
Ammonium Bromide Salt Formula
Ammonium bromide is considered an inorganic salt due to its ionic nature. As mentioned earlier, its chemical formula is NH₄Br, which denotes the combination of ammonium and bromide ions. Salts like ammonium bromide are widely used in various chemical processes, including photography, pharmaceuticals, and as a flame retardant.
Cetyl Trimethyl Ammonium Bromide Formula
Cetyl trimethyl ammonium bromide, often CTAB, is a quaternary ammonium compound. Its chemical formula is C₁₉H₄₂BrN, which includes a cetyl group (C₁₉H₄₂), three methyl groups (CH₃), and a bromine atom (Br) attached to a nitrogen atom (N). CTAB has diverse applications, ranging from its use in the synthesis of nanoparticles to acting as a surfactant in various chemical and biological processes. The ammonium bromide formula and its various aspects is essential for chemists and researchers across multiple fields. Whether investigating its chemical structure, its behavior in aqueous solutions, or exploring related compounds like cetyl trimethyl ammonium bromide, these insights contribute to a broader understanding of the chemistry and applications of these compounds.
Properties of Ammonium Bromide
Ammonium bromide is an inorganic chemical compound with a range of properties that make it useful in various applications. Here are some of the key properties of ammonium bromide:- Chemical Formula: The chemical formula of ammonium bromide is NH₄Br, which consists of the ammonium ion (NH₄⁺) and bromide (Br⁻). The combination of these ions forms this ionic compound.
- Physical State: Ammonium bromide is typically found as a white crystalline solid at room temperature. It can also exist in powder form.
- Solubility: Ammonium bromide is highly soluble in water, readily dissolving in aqueous solutions. This property is often utilized in various chemical processes and applications.
- Melting Point: The compound has a relatively low melting point, typically around 452°C (845.6°F). This characteristic is advantageous in applications where the substance needs to be easily melted or dissolved.
- Boiling Point: Ammonium bromide decomposes upon heating, and it does not have a well-defined boiling point. Instead, it undergoes thermal decomposition, giving off ammonia gas and leaving behind bromine.
- Odour: Ammonium bromide has a slight ammonia-like odour, a common characteristic of ammonium compounds. It is not as pungent as ammonia itself.
- Electrical Conductivity: When dissolved in water, ammonium bromide dissociates into its constituent ions (NH₄⁺ and Br⁻). These ions are capable of conducting electricity, making the solution electrolytic.
- Hygroscopic: Ammonium bromide is hygroscopic, meaning it can absorb moisture from the air. This property can affect the stability and handling of the compound, especially in humid environments.
| Related Links | |
| Glutaric Acid Formula | Pentane formula |
| Potassium Acetate formula | Oxalic Acid formula |
Ammonium Bromide FAQs
How do you write ammonium bromide?
What is the formula name for ammonium bromide?
What is NH4Br used for?
What is the chemical formula for ammonium bromide Class 9?










