
Sodium citrate , also known as E number E 331, is a compound derived from the alkalization of citric acid. It is available in three forms: monosodium citrate, disodium citrate, and trisodium citrate. However, the term sodium citrate typically refers to trisodium citrate. Its chemical formula is Na 3 C 6 H 5 O 7 and has extensive applications in medicine and the food industry.
What is Sodium Citrate?
Sodium citrate, also known as E number E 331, is a compound derived from the alkalization of citric acid. It is available in three forms: monosodium citrate, disodium citrate, and trisodium citrate. However, the term sodium citrate typically refers to trisodium citrate. Its chemical formula is Na 3 C 6 H 5 O 7 and has extensive applications in medicine and the food industry.
Sodium Citrate Structure
Sodium is a metal, while citric acid is a group of nonmetals called polyatomic ions. Therefore, both chemical entities are interconnected.
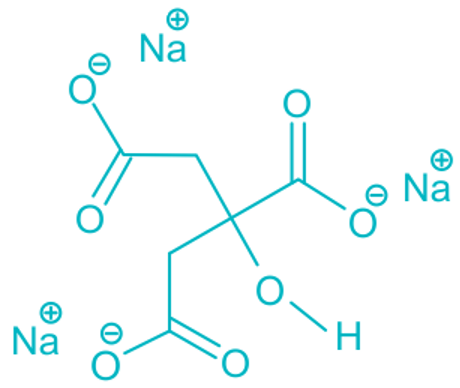
In a bath bomb, citric acid reacts with sodium bicarbonate in the presence of water to form this chemical.
C 6 H 8 O 7 + 3NaHCO 3 + H 2 O ⇢ Na 3 C 6 H 5 O 7 + 3H 2 O + 3CO 2
Also Check – Iron (III) Hydroxide Formula
Properties of Sodium Citrate
- The molecular formula of sodium citrate is Na 3 C 6 H 5 O 7 , with a molecular weight of 258.068 g/mol.
- It appears as a white crystalline powder or granular crystals and is soluble in water.
- When exposed to moist air, it becomes deliquescent. However, it is insoluble in alcohol and has a sour taste reminiscent of citric acid.
- With seven hydrogen bond acceptors and one hydrogen bond donor, it has a monoisotopic mass of 257.973 g/mol.
- Sodium citrate's melting point applications of Sodium Citrate
Also Read – Malic Acid Formula
Applications of Sodium Citrate
Here are some of the major applications of sodium citrate in food and beverage, healthcare, and industry.
Food and Beverages
Processed cheese products use sodium citrate as an emulsifying salt, facilitating the melting of cheese without becoming greasy. In combination with citric acid, it functions as a buffering agent, providing accurate pH control for a wide range of foods and beverages. Food additives are mainly used as flavorings, to enhance the taste, or for preservation.
Also Read: Molar Volume Formula
Healthcare
Sodium citrate is an extremely useful compound with a variety of applications. This anticoagulant is commonly utilized for both blood collection and storage. In addition to its role in urinary tract infections, it can also serve as a reliable laxative and assist with acidosis. Notably, sodium citrate is a key component in the World Health Organization's oral rehydration solution. It also serves as an effective antacid, particularly prior to receiving anaesthesia. You'll find this versatile ingredient in numerous pharmaceuticals, dyes, cosmetics, and deodorants. It's also frequently incorporated into hair care, oral care, skincare, and bathing products.
Industrial Applications
In addition to clearing steam blocks, hot water systems of calcium and rust layers, citric acid also serves as a buffering and complexing agent for electroplating processes. Due to citric acid's chelating properties and non-toxicity, it is beneficial to the textile and building industries.
Sodium Citrate Formula FAQs
Q1. What is the chemical formula of sodium citrate?
Q2. What is the purpose of sodium citrate in food?
Q3. Is sodium citrate safe to consume?
Q4. Can sodium citrate be used in medical applications?
Q5. What are some other uses of sodium citrate?










