The Barium Hydroxide Formula Ba(OH) 2 , or barium hydroxide or baryta, is a white, odourless powder with potentially harmful properties. In solution, it exhibits ionic behaviour by producing two hydroxide ions per molecule. This makes it the ideal reagent for carboxamide metalization. It should be noted that barium hydroxide is less destructive than barium oxide in this process.
Barium Hydroxide Formula and Structure
Barium hydroxide is a chemical compound with the formula Ba(OH) 2 . This formula indicates that a single molecule of barium hydroxide consists of one barium ion (Ba²⁺) and two hydroxide ions (OH⁻). The hydroxide ions, in turn, consist of one oxygen (O) atom and one hydrogen (H) atom, giving them a charge of -1.
Ba(OH) 2 is the chemical formula for barium hydroxide. It is available as anhydrous, monohydrate, and octal hydrate, with molar masses of 171.34 g mol-1, 189.955 g mol-1, respectively. The structure of the compound is formed with 2 hydroxyl anions, (OH–) and 1 barium cation (Ba2+).
In addition, its chemical structure is shown below as an image, in the form of the common representations we use for organic molecules if it is hydrated.
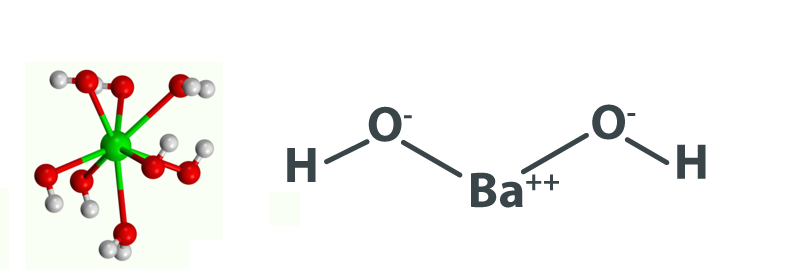
Barium Hydroxide Formula by Criss-Cross Method
The criss-cross method is a technique used to determine the chemical formula of an ionic compound. In the case of barium hydroxide, this method involves crossing the numerical charges of the ions to find the subscripts for each element. Since barium has a charge of +2 (Ba²⁺) and hydroxide has a charge of -1 (OH⁻), you would write the formula as Ba 2 (OH) 2 , which simplifies to Ba(OH) 2 .
Barium Hydroxide Formula Name
The name of the compound Ba(OH) 2 is simply "barium hydroxide." It accurately reflects the chemical composition of the substance, with "barium" representing the metal cation (Ba²⁺) and "hydroxide" denoting the polyatomic anion (OH⁻).
Barium Hydroxide Formula Mass
Consider its constituent elements' atomic masses to calculate the formula mass of barium hydroxide (Ba(OH) 2 ). Barium (Ba) has an atomic mass of approximately 137.33 g/mol, while the atomic mass of oxygen (O) is about 16.00 g/mol, and hydrogen (H) has an atomic mass of approximately 1.01 g/mol. To find the formula mass, add these values together, accounting for the two hydroxide ions: 137.33 g/mol (Ba) + 2 * (16.00 g/mol (O) + 1.01 g/mol (H)) = 171.36 g/mol.
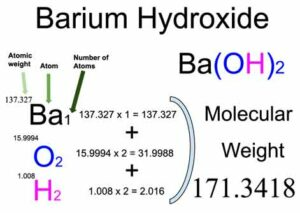
Barium Hydroxide Formula Units
The formula Ba(OH) 2 represents one formula unit of barium hydroxide. Each formula unit contains one barium ion (Ba²⁺) and two hydroxide ions (OH⁻).
Barium Hydroxide Formula, Including Charges
The formula Ba(OH) 2 includes the charges of the ions. Barium (Ba²⁺) carries a charge of +2, while hydroxide (OH⁻) has a charge of -1. This balanced combination of charges ensures that the compound is electrically neutral.
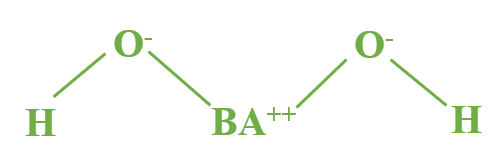
Barium Hydroxide Formula Hydrate
Barium hydroxide can also be hydrated as barium hydroxide octahydrate (Ba(OH)2·8H2O). This compound contains eight water molecules (H2O) in its structure. The presence of water molecules can affect the compound's properties and reactivity.
Barium Hydroxide Formula Salt
Barium hydroxide is indeed a salt. Salts are ionic compounds formed by combining a metal cation (in this case, barium, Ba²⁺) and a non-metal anion (the hydroxide, OH⁻). Salts are typically solid at room temperature and have distinct chemical and physical properties.
Physical Properties of Barium Hydroxide Formula
With a white colour and no odour, barium hydroxide is solid. It has a density of 3.743 g mL-1 (monohydrate form) and 2.18 g mL-1 (octahydrate form). It has melting points of 78 C (octahydrate form), 300 C (monohydrate form), and 407 C (anhydrous form). At low temperatures, all 3 forms are slightly soluble in water, but as the temperature increases, solubility in water increases.
Chemical Properties of Barium Hydroxide Formula
As a result of the release of hydroxyl anions when barium hydroxide is dissolved in water, we obtain alkali solutions. Because it can react with sulphuric, phosphoric, and other acids to produce the respective salts, such as barium phosphate, barium hydroxide can be used to make barium salts.
| Related Links | |
| Acetone formula | Ascorbic Acid formula |
| Actetamide formula | Butane Formula |
Barium Hydroxide Formula FAQs
How is barium hydroxide used?
What is the chemical name of barium hydroxide?
Is barium hydroxide a base or acid?
Is Ba(OH)2 a formula unit?