
CBSE Class 11 Biology Notes Chapter 9: In CBSE Class 11 Biology Notes Chapter 9 about Biomolecules, you learn about the important molecules that make life possible. These molecules, like carbohydrates, lipids, proteins, and nucleic acids, are essential for many functions in living things.
By studying these biomolecules, you get a better understanding of how living organisms work on a molecular level. The chapter explains these molecules in simple terms, making it easier for you to grasp their roles and importance in biology.CBSE Class 11 Biology Notes Chapter 9 Biomolecules Overview
Chapter 9 of CBSE Class 11 Biology, "Biomolecules," explores the diverse molecules essential for life processes. Key biomolecules include carbohydrates, proteins, lipids, and nucleic acids, each with specific structures and functions. Carbohydrates provide energy, while proteins are crucial for structure and enzyme functions. Lipids store energy and make up cell membranes, whereas nucleic acids (DNA and RNA) hold genetic information. The chapter explains the monomers and polymers of these molecules, and the biochemical reactions they undergo, such as hydrolysis and dehydration synthesis. Enzymes, as biological catalysts, facilitate these reactions, ensuring efficient cellular functioning and metabolism.CBSE Class 11 Biology Notes Chapter 9 Biomolecules PDF
You can access the biomolecules class 11 notes PDF through the link provided below. This document contains valuable information about biomolecules, which are essential for life processes. It covers various topics such as carbohydrates, proteins, lipids, and nucleic acids, explaining their structures, functions, and significance in living organisms. Whether you're a student or someone interested in biology, this PDF will help you understand the basics of biomolecules and their role in biological systems.CBSE Class 11 Biology Notes Chapter 9 PDF
CBSE Class 11 Biology Notes Chapter 9 Biomolecules
Below, you'll find the biomolecules class 11 notes. These notes cover various questions and exercises related to the chapter, helping you understand the concepts of biomolecules effectively. By going through these notes, you can clarify your doubts, reinforce your understanding, and prepare well for exams. Whether you're a student studying for exams or someone interested in learning about biomolecules, these solutions will be beneficial in enhancing your knowledge and problem-solving skills.Biomolecules
 Biomolecules are crucial organic compounds produced by living organisms, serving essential functions and forming the foundation of life itself. These molecules consist of carbon, nitrogen, hydrogen, phosphorus, oxygen, and sulfur. Among the various types of biomolecules, four are particularly common: proteins, carbohydrates, nucleic acids, and lipids. Each of these biomolecules plays a distinct role in biological processes, contributing to the overall functioning and structure of living organisms.
Biomolecules are crucial organic compounds produced by living organisms, serving essential functions and forming the foundation of life itself. These molecules consist of carbon, nitrogen, hydrogen, phosphorus, oxygen, and sulfur. Among the various types of biomolecules, four are particularly common: proteins, carbohydrates, nucleic acids, and lipids. Each of these biomolecules plays a distinct role in biological processes, contributing to the overall functioning and structure of living organisms.
Analysis of chemical composition
When analyzing the chemical composition of living tissues, different methods are employed based on whether the compounds being studied are organic or inorganic. For organic compounds, such as proteins, nucleic acids, and carbohydrates, the tissues are treated with trichloroacetic acid. This treatment involves grinding the tissues to create a slurry, which aids in breaking down the organic matter for analysis. On the other hand, when analyzing the inorganic chemical composition, such as minerals and metals, the tissues are burned to form ashes. This process involves heating a sample of tissue until it combusts completely, leaving behind only the inorganic components as ash. These methods help scientists understand the chemical makeup of living tissues and provide valuable insights into their biological functions.Proteins
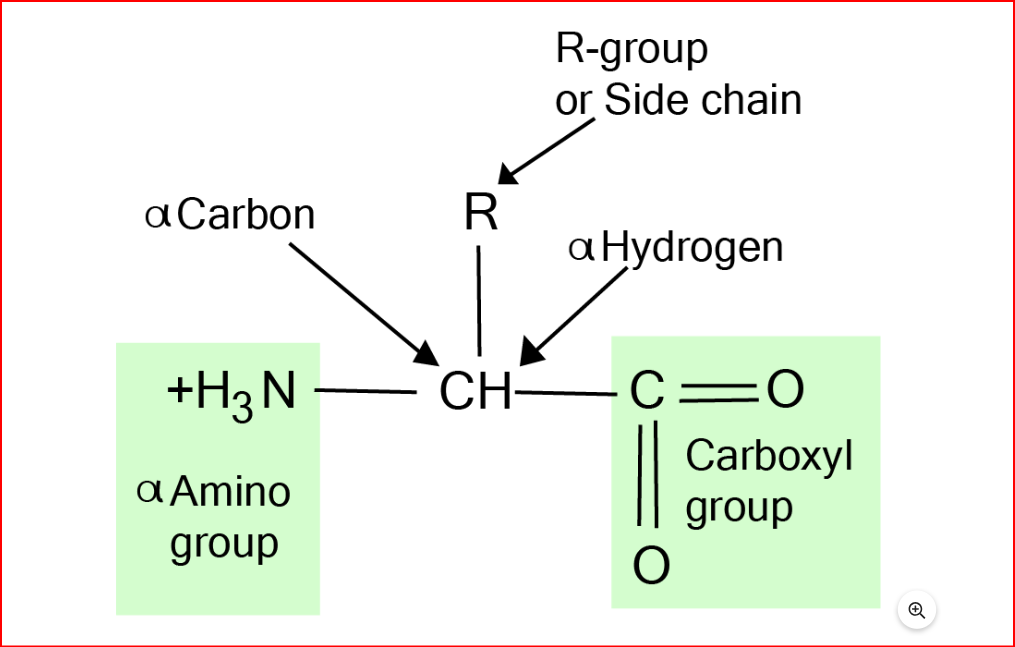 In living organisms, there are crucial substances known as proteins, which are a type of nitrogenous organic compounds made up of long chains of amino acids. Amino acids serve as the building blocks of proteins, and there are approximately 22 different types found in nature, consisting of hydrogen, carbon, oxygen, and nitrogen. Each amino acid has an amino group, a hydrogen atom, a carboxyl group, and a unique side chain bonded to the alpha-carbon.
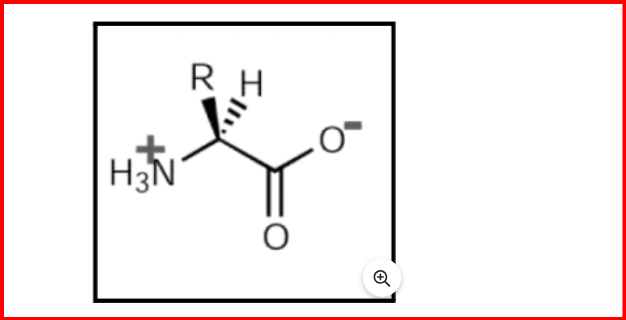
When dissolved in water, amino acids exist as dipolar ions or zwitterions, capable of either donating or accepting protons. They exhibit optical activity, meaning they can rotate polarized light, except for glycine, as they contain a chiral carbon, which is a carbon atom with four different substituents.
In living organisms, there are crucial substances known as proteins, which are a type of nitrogenous organic compounds made up of long chains of amino acids. Amino acids serve as the building blocks of proteins, and there are approximately 22 different types found in nature, consisting of hydrogen, carbon, oxygen, and nitrogen. Each amino acid has an amino group, a hydrogen atom, a carboxyl group, and a unique side chain bonded to the alpha-carbon.
When dissolved in water, amino acids exist as dipolar ions or zwitterions, capable of either donating or accepting protons. They exhibit optical activity, meaning they can rotate polarized light, except for glycine, as they contain a chiral carbon, which is a carbon atom with four different substituents.
Structure of amino acid
 Amino acids can combine to form peptides, with two amino acids creating a dipeptide, three forming a tripeptide, and 12 to 20 linking to form an oligopeptide chain. When numerous amino acids join together, they create polypeptides. The first amino acid in a polypeptide chain is called the N terminal or amino-terminal, while the last one is termed the C terminal or carboxyl-terminal.
Amino acids can combine to form peptides, with two amino acids creating a dipeptide, three forming a tripeptide, and 12 to 20 linking to form an oligopeptide chain. When numerous amino acids join together, they create polypeptides. The first amino acid in a polypeptide chain is called the N terminal or amino-terminal, while the last one is termed the C terminal or carboxyl-terminal.
Formation of peptide bond
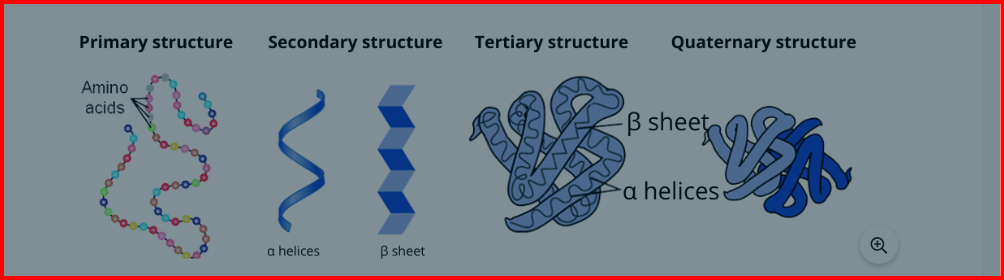 Proteins exhibit four levels of organization, each contributing to their overall structure and function:
Proteins exhibit four levels of organization, each contributing to their overall structure and function:
Primary Structure : This level refers to the linear sequence of amino acids linked together by peptide bonds.
Secondary Structure : At this stage, the protein begins to fold into more complex shapes, typically forming alpha-helices or beta-sheets. These structures are stabilized by hydrogen bonds between the carbonyl and amino groups along the polypeptide backbone.
Tertiary Structure : This level involves the three-dimensional arrangement of the entire protein molecule, including interactions between amino acid side chains. Stabilizing forces such as hydrophobic interactions, hydrogen bonds, electrostatic interactions, van der Waals forces, and covalent bonds help maintain the structure.
Quaternary Structure : Some proteins consist of multiple polypeptide chains that come together to form a functional protein complex. The interactions between these individual chains, such as hydrophobic interactions and hydrogen bonds, contribute to the quaternary structure. Examples include hemoglobin, which is composed of four subunits.
Levels of protein organization
Proteins can also be classified into two main categories based on their shapes and solubility:- Fibrous Proteins : These proteins have an elongated, rod-like shape and are insoluble in water. They primarily serve structural and protective roles in the body.
- Globular Proteins : These proteins have a more spherical shape and are soluble in water. They perform a wide range of functions, including enzymatic activity, transport, and immune response.
Nucleic acids
Nucleic acids, discovered by Friedrich Miescher, are another essential class of biomolecules. There are two main types of nucleic acids: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are composed of nucleotides, which consist of three components: a nitrogenous base, a five-carbon sugar (deoxyribose in DNA and ribose in RNA), and a phosphate group.Structure of nucleotide
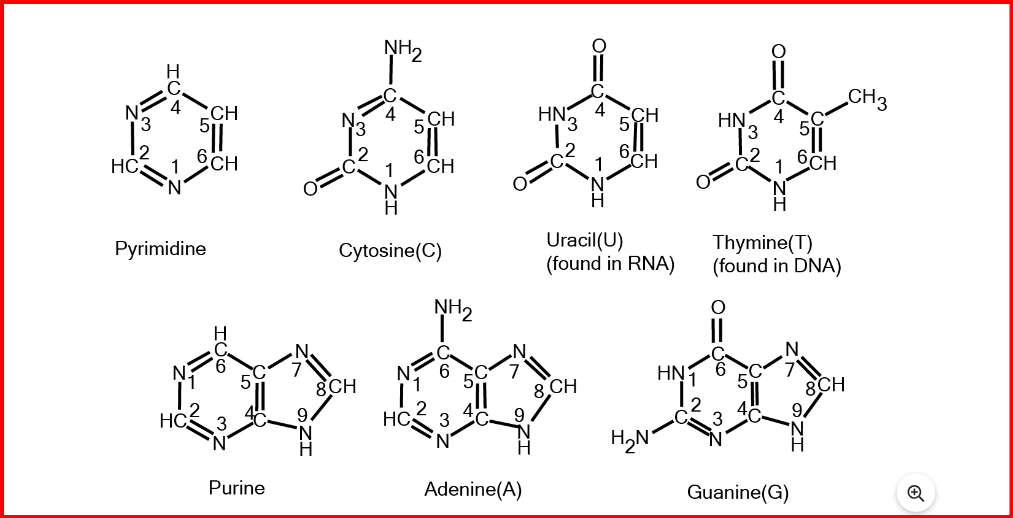 The nitrogenous bases found in nucleic acids are aromatic molecules with a heterocyclic structure. There are two main types of nucleic acids: purines and pyrimidines.
Purines include adenine and guanine, while pyrimidines include thymine (in DNA), cytosine, and uracil (in RNA).
DNA, or deoxyribonucleic acid, contains deoxyribose, a 5-carbon sugar, while RNA, or ribonucleic acid, contains ribose. When a nucleoside (which consists of a nitrogenous base and a sugar) is combined with a phosphate group, it forms a nucleotide.
The nitrogenous bases found in nucleic acids are aromatic molecules with a heterocyclic structure. There are two main types of nucleic acids: purines and pyrimidines.
Purines include adenine and guanine, while pyrimidines include thymine (in DNA), cytosine, and uracil (in RNA).
DNA, or deoxyribonucleic acid, contains deoxyribose, a 5-carbon sugar, while RNA, or ribonucleic acid, contains ribose. When a nucleoside (which consists of a nitrogenous base and a sugar) is combined with a phosphate group, it forms a nucleotide.
Structure of Double-Stranded DNA
There are two main types of DNA structures:B DNA:
- The long polynucleotide strands coil around an axis.
- The two strands are arranged in an antiparallel manner, meaning they run in opposite directions.
- Adenine nucleoside base pairs with thymine, while guanine base pairs with cytosine.
- Adenine and thymine are held together by two hydrogen bonds, while guanine and cytosine are held together by three hydrogen bonds.
Z DNA:
- This DNA structure is thinner compared to B DNA.
- The purine and pyrimidine bases are arranged alternately.
- The DNA has a high salt concentration to stabilize its structure.
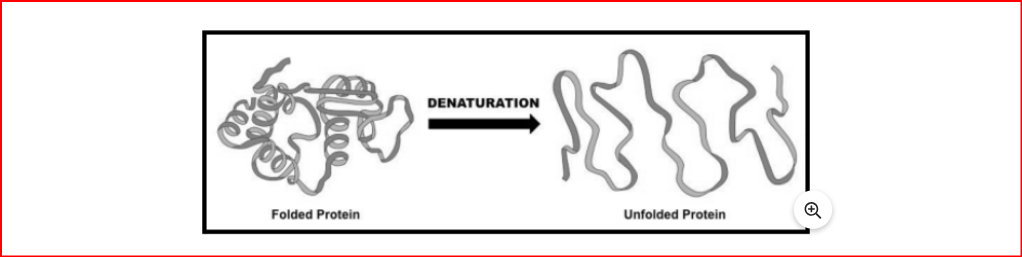 Denaturation of DNA occurs when the two strands separate due to exposure to varying pH, temperature, etc. To restore the original DNA structure, denaturants like pH and temperature need to be removed, a process known as renaturation or annealing.
Denaturation of DNA occurs when the two strands separate due to exposure to varying pH, temperature, etc. To restore the original DNA structure, denaturants like pH and temperature need to be removed, a process known as renaturation or annealing.
RNA
RNA, or ribonucleic acid, is single-stranded and found in various forms within the cell: (i) Messenger RNA (mRNA): Carries genetic information from DNA to the next generation in the form of codons (three nucleotides form a codon), aiding in protein synthesis. (ii) Transfer RNA (tRNA): Facilitates protein synthesis by interacting between the language of nucleic acid and protein, located in the cytoplasm. (iii) Ribosomal RNA (rRNA): Constitutes ribosomes, crucial for protein synthesis in both eukaryotes and prokaryotes.Carbohydrates
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen, including polyhydroxy aldehydes or polyhydroxy ketones. Among them, monosaccharides like glucose are the simplest carbohydrates. Disaccharides consist of two monosaccharides joined together, while oligosaccharides are formed by polymerizing 2 to 10 units of monosaccharides. Polysaccharides, on the other hand, are formed when hundreds to thousands of monosaccharides are joined together. Monosaccharides with an aldehyde group are aldoses, while those with a ketone group are ketoses. Trioses are the simplest monosaccharides.Some common saccharides
Starch, a common saccharide, stores energy in plants. It is composed of several units of glucose and has a branched structure. Starch comprises two different polymers: amylose and amylopectin. Amylose is an unbranched polymer of glucose units, while amylopectin consists of branched polymer chains of glucose units.Structure of starch
Energy Storage: Lipids are a primary storage form of energy in organisms. They can be stored in adipose tissue and mobilized when energy is needed, providing a long-term energy reserve.
Structural Component of Membranes: Lipids, particularly phospholipids, are essential components of cell membranes. They form the lipid bilayer, which provides structural integrity to cell membranes and regulates the passage of molecules in and out of cells.
Protection: In addition to providing structural support, certain lipids act as protective barriers. For example, lipids found in the skin help prevent water loss and protect against pathogens. In plants, lipids in the form of waxes provide a protective coating on leaves and stems, reducing water loss and protecting against pests and pathogens.
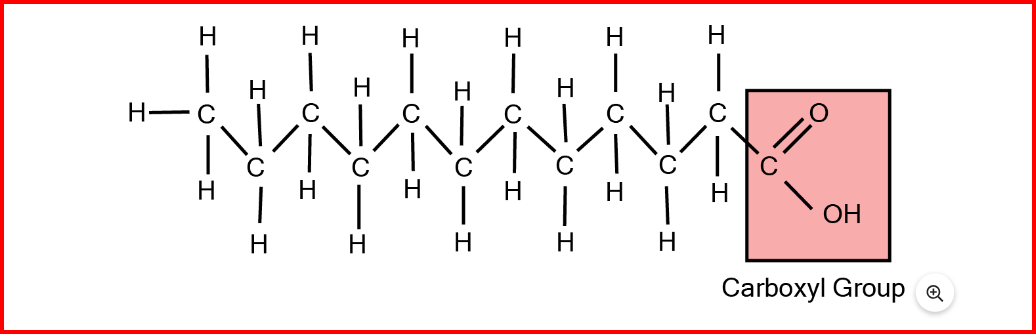
Fatty Acids
CBSE Class 11 Biology Notes Chapter 9 FAQs
What are biomolecules?
What are monosaccharides, disaccharides, and polysaccharides?
How are proteins structured, and what are their functions?
What are nucleic acids, and what role do they play?
Why are biomolecules essential for living organisms?










