CBSE Class 11 Physics Notes Chapter 11: In CBSE Class 11 Physics, Chapter 11 is all about Thermal Properties of Matter. This chapter teaches us about heat and temperature, how things expand when they're heated, how much heat different materials can hold, and how we measure heat.
We also learn about how heat moves from one object to another and how we can use this knowledge in everyday life. By studying this chapter, students will understand how heat affects the things around us and how we can measure and use it in practical ways.CBSE Class 11 Physics Notes Chapter 11 Thermal Properties of Matter PDF
You can find the PDF link for CBSE Class 11 Physics Notes Chapter 11 on Thermal Properties of Matter below. This document contains detailed explanations and helpful insights into the concepts covered in the chapter. It is a valuable resource for students to enhance their understanding of heat, temperature, thermal expansion, and other related topics. By using these notes, students can effectively prepare for their exams and improve their performance in physics.CBSE Class 11 Physics Notes Chapter 11 Thermal Properties of Matter PDF
CBSE Class 11 Physics Notes Chapter 11 Thermal Properties of Matter
Thermal Properties of Matter
Thermal properties encompass characteristics of a material related to its ability to conduct heat. Essentially, these are the traits exhibited by a substance when heat flows through it. These properties fall within the broader scope of the physical properties of materials. When a material encounters fluctuations in temperature—be it excessive heat or extreme cold—its thermal properties dictate its response. The key components of thermal properties include:- Heat Capacity
- Thermal Expansion
- Thermal Conductivity
- Thermal Stress
Where is the amount of heat transferred, is the mass, is the specific heat capacity, and is the change in temperature.
Thermal Expansion
Thermal expansion is a phenomenon where materials increase in size as their temperature rises. When heat is applied to a substance, its particles start to move more vigorously, causing them to spread out and take up more space. This expansion can be observed in various everyday objects, such as metal rods, bridges, and even liquids like water. It is a crucial concept in engineering and construction, as failing to account for thermal expansion can lead to structural damage or malfunctioning of machinery. Thermal expansion is quantified by the coefficient of thermal expansion, which measures how much a material's dimensions change per unit temperature increase. Understanding thermal expansion helps engineers design structures and devices that can withstand temperature changes without buckling or breaking.Thermal Conductivity
Thermal conductivity refers to the ability of a material to conduct heat. In simpler terms, it's a measure of how easily heat can move through a substance. Materials with high thermal conductivity, such as metals like copper and aluminum, allow heat to flow through them quickly. On the other hand, materials with low thermal conductivity, like wood or plastic, hinder the transfer of heat. Understanding thermal conductivity is essential in various applications, from designing efficient heat exchangers and insulating materials to optimizing the performance of electronic devices. Engineers and scientists use thermal conductivity values to select the appropriate materials for specific purposes and to enhance the efficiency of thermal systems.Thermal stress
Thermal stress refers to the internal stresses that develop within a material due to changes in temperature. When a material is subjected to temperature variations, its particles expand or contract, causing internal forces to arise. These forces can lead to deformation, cracking, or even structural failure if the material is unable to withstand the stress. Thermal stress is particularly significant in materials with different coefficients of thermal expansion, such as metals and ceramics, as the uneven expansion or contraction of different parts can create internal strains. Engineers and material scientists must consider thermal stress when designing structures, components, and systems to ensure their integrity and durability under varying temperature conditions. Strategies such as using materials with compatible thermal expansion coefficients or incorporating thermal expansion joints help mitigate the effects of thermal stress in engineering applications.Heat and Calorimetry
Heat and calorimetry are fundamental concepts in thermodynamics that relate to the transfer and measurement of heat. Heat refers to the transfer of thermal energy from a hotter object to a cooler one. It is a form of energy that flows spontaneously from regions of higher temperature to regions of lower temperature until thermal equilibrium is reached. Understanding heat transfer mechanisms is essential in various fields, including engineering, physics, and chemistry, as it influences the behavior of materials and systems. Calorimetry, on the other hand, is the science of measuring heat changes during physical and chemical processes. It involves using calorimeters, devices designed to isolate the system undergoing the temperature change and accurately measure the heat exchanged with the surroundings. Calorimetry is used to determine the specific heat capacity of substances, study chemical reactions, and quantify energy changes in various processes. Together, heat and calorimetry play crucial roles in understanding and analyzing thermal phenomena, facilitating advancements in science, engineering, and technology.Celsius and Fahrenheit Temperature Scale
The Celsius and Fahrenheit temperature scales are two common systems used to measure temperature. Celsius, also known as Centigrade, is a metric system unit of temperature measurement. It is based on dividing the temperature range between the freezing and boiling points of water into 100 equal intervals. In this scale, water freezes at 0 degrees Celsius (°C) and boils at 100 degrees Celsius (°C) under standard atmospheric pressure. Fahrenheit is a temperature scale commonly used in the United States and a few other countries. It divides the temperature range between the freezing and boiling points of water into 180 equal intervals. In this scale, water freezes at 32 degrees Fahrenheit (°F) and boils at 212 degrees Fahrenheit (°F) under standard atmospheric pressure.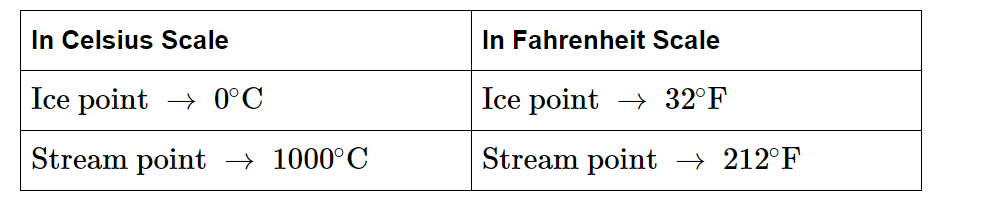
Thermometers
A thermometer is a tool utilized to gauge the temperature of a system. Various types of thermometers exist, including liquid-in-glass thermometers, platinum resistance thermometers, and constant volume gas thermometers. Liquid-in-glass and platinum resistance thermometers offer consistent readings for the ice point and steam point. However, their readings may differ for other liquids and materials. Contrarily, a constant volume gas thermometer yields consistent readings irrespective of the gas utilized. This type of thermometer relies on the principle that, at low pressures and constant volume, the product of pressure (P) and temperature (T) remains constant for a gas.Coefficient of Volume Expansion of Cu as a Function of Temperature
For ideal gases, γ 𝛾 is inversely related to the temperature at constant pressure.
V = nRT P V = nRTP
⇒ Δ V V = Δ T T ⇒ΔVV = ΔTT
⇒ γ = ⊥ T ⇒ 𝛾 = ⊥T
Water, on the other hand, contracts when heated from 0 ∘ C to 4 ∘ C 0 ∘ C to 4 ∘ C and thus its density rises from 0 ∘ C to 4 ∘ C 0 ∘ C to 4 ∘ C. This is known as anomalous expansion.
In general γ = 3 α = 3 2 β 𝛾 = 3 𝛼 = 32 𝛽
Proof: Consider a cube of length l that expands equally in all directions when its temperature rises by a small
We have
Also,
Δ V = ( l Δ l ) 3 l 3 ΔV= (𝑙Δ𝑙)3 𝑙3
= l 3 + 3 l 2 Δ l + 3 l Δ l 2 + Δ l 2 l 3 = 𝑙3 + 3𝑙2Δ𝑙 + 3𝑙Δ𝑙2 + Δ𝑙2 𝑙3
= 3 l 2 Δ l . . . ( 1 ) = 3𝑙2Δ𝑙 ...(1)
In Equation (1) we ignore 3 l Δ l 2 & Δ l 3 as Δ l 3𝑙Δ𝑙2 &Δ𝑙3 as Δ𝑙 is very small as compared to l.
So, Δ V = 3 V l Δ l ΔV = 3V𝑙Δ𝑙 = 3 V α Δ T [ Using V l = l 2 ] ⋯ ⋯ ( 2 ) =3V 𝛼 Δ T [Using V𝑙= 𝑙2]⋯⋯(2) Δ V V = 3 α Δ T ΔVV = 3 𝛼 Δ T
Similarly, we can prove for area expansion coefficient of thermal expansion is prevented inside the rod by rigidly fixing its ends, then the rod acquires a compressive strain due to external fones at the ends. The corresponding stress set up in the rod is called thermal stress.
Hence, thermal stress = F A = Y ( L − L 0 ) / L 0 =𝐹𝐴=𝑌(𝐿−𝐿0)/𝐿0 where Y is Young's modulus of the given material.
This can be simplified into Y ( α Δ T ) / L 0 𝑌(𝛼Δ𝑇)/𝐿0 .
Practical applications include railway tracks, metal tyres on cart wheels, bridges, and a variety of other structures.
Benefits of CBSE Class 11 Physics Notes Chapter 11
CBSE Class 11 Physics Notes Chapter 11 on Thermal Properties of Matter offer numerous benefits to students:Comprehensive Understanding : These notes provide a detailed explanation of key concepts related to thermal properties, helping students develop a thorough understanding of the topic.
Clarity and Organization : The notes are structured in a clear and organized manner, making it easier for students to follow along and grasp complex concepts.
Revision Aid : They serve as an effective tool for revision, allowing students to quickly review important points before exams or assessments.
Exam Preparation : With concise summaries and illustrative examples, these notes aid in exam preparation by highlighting essential topics and concepts likely to be tested.
Supplementary Learning : They complement classroom lectures and textbooks, offering additional insights and explanations to enhance learning.
CBSE Class 11 Physics Notes Chapter 11 FAQs
What are thermal properties of matter?
Why are thermal properties important?
What is specific heat capacity?
How do I calculate thermal stress?