Entropy is a fundamental concept in thermodynamics that represents the degree of disorder or randomness in a system. It is a measure of the number of possible ways energy can be distributed among the particles in a system. In simpler terms, entropy quantifies the amount of chaos, randomness, or uncertainty in a system. It increases when a system transitions from an ordered state to a more disordered state, and it tends to increase over time in isolated systems, in accordance with the second law of thermodynamics.
Maxwell Law of Distribution of Molecules
The Maxwell-Boltzmann distribution, often referred to as the Maxwell Law of Distribution of Molecules, describes the distribution of speeds of particles in a gas at a given temperature. It states that the speeds of particles in a gas follow a probability distribution, with the majority of particles having speeds near the average, and fewer particles having speeds that are either much higher or much lower than the average.
Brownian Motion
Brownian motion is the random motion of particles suspended in a fluid (liquid or gas), resulting from their collisions with the fast-moving atoms or molecules in the fluid. This phenomenon was first observed by the Scottish botanist Robert Brown in 1827 when he observed pollen grains suspended in water. He noticed that the pollen grains moved randomly, even though there was no external force acting on them.
Brownian motion occurs because the particles in the fluid are constantly moving due to their thermal energy. When these fast-moving fluid particles collide with the suspended particles, they transfer momentum to them, causing them to move erratically. This motion is characterized by random fluctuations in position and is often described as a "random walk."
Isobaric Process
An isobaric process is a thermodynamic process that occurs at constant pressure. During an isobaric process, the pressure of the system remains unchanged while other properties, such as volume, temperature, and internal energy, may vary. Isobaric processes are commonly encountered in various natural and engineering systems, such as in the operation of a gas turbine or in the heating of a substance in an open container.
Boiling Process
During the boiling process,
Internal energy,
Q
=
m
L
V
𝑄=𝑚𝐿𝑉,
where
L
V
𝐿𝑉
is the latent heat of vaporization.
Work done,
W
=
P
[
V
2
V
1
]
𝑊=𝑃[𝑉2𝑉]
(Pressure is constant during boiling and it is equal to atmospheric pressure)
With respect to the first law of thermodynamics,
Δ
U
=
Q
−
W
Δ𝑈=𝑄−𝑊
⇒
Δ
U
=
m
L
f
−
P
(
V
2
−
V
1
)
Refrigerator and Heat Pumps
Refrigerators and heat pumps are both devices that transfer heat from one location to another, but they operate in different ways and serve different purposes.
A refrigerator is a device used to cool enclosed spaces or substances, typically to lower temperatures than the surroundings. It operates by removing heat from the interior of the refrigerator and transferring it to the surrounding environment. This process is achieved through the use of a refrigeration cycle, which typically involves the compression and expansion of a refrigerant fluid.
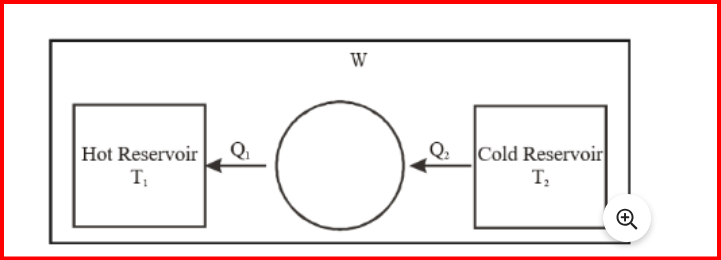
On the other hand, a heat pump is a device used to transfer heat from one location to another. Unlike a refrigerator, which only removes heat, a heat pump can both remove heat from a cooler space and deliver it to a warmer space. Heat pumps are commonly used for heating and cooling buildings, as well as for heating water and other fluids. They operate using a similar refrigeration cycle but can be reversed to provide either heating or cooling, depending on the desired outcome.
Refrigerators are primarily used for cooling, while heat pumps are used for both heating and cooling. Both devices rely on the principles of thermodynamics and the refrigeration cycle to transfer heat effectively.
Carnot Engine
Sadi Carnot devised an ideal cycle of operation for a heat engine termed the Carnot cycle.
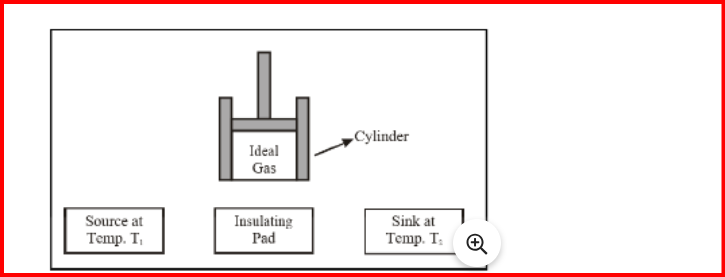
The engine used for realizing this ideal cycle is known as the Carnot heat engine.
Carnot Theorem
Statement:
When operating between two specified temperatures,
𝑇1
T
1
of the hot reservoir (the source) and
𝑇2
T
2
of the cold reservoir (the sink), no engine can achieve greater efficiency than the Carnot engine.
Proof:
Step 1:
Let's consider a reversible engine, labeled as R, and an irreversible engine, labeled as I, operating between the source (hot reservoir at
𝑇1
T
1
) and the sink (cold reservoir at
𝑇2
T
2
).
Step 2:
We'll pair up these two engines so that I functions as a heat engine and R operates as a refrigerator.
Step 3:
Suppose engine I absorbs heat
𝑄1
Q
1
from the source, delivers work
𝑊1
W
1
, and releases the remaining heat
𝑄1−𝑊1
Q
1
−
W
1
to the sink during a single cycle.
Step 4:
Engine R is set up to return the same amount of heat to the source, extracting
𝑄2
Q
2
from the sink and requiring work
𝑊=𝑄1−𝑄2
W
=
Q
1
−
Q
2
to be done on it.
Step 5:
Let's assume that the efficiency of engine R (
𝜂𝑅
η
R
) is less than that of engine I (
𝜂𝐼
η
I
), implying that when R acts as an engine, it yields less work output than I. Consequently,
𝑊<𝑊1
W
<
W
1
for a given
𝑄1
Q
1
, and
𝑄1−𝑊>𝑄1−𝑊1
Q
1
−
W
>
Q
1
−
W
1
.
Step 6:
In this scenario, the I-R system would extract
(𝑄1−𝑊)−(𝑄1−𝑊1)=𝑊1−𝑊
(
Q
1
−
W
)
−
(
Q
1
−
W
1
)
=
W
1
−
W
amount of heat and deliver the same amount of work in a single cycle without any change in the source or elsewhere. This contradicts the second law of thermodynamics (Kelvin-Planck statement).
Thus, the assumption that
𝑄1>𝑄𝑅
Q
1
>
Q
R
is incorrect.
A similar argument can be established for the second statement of the Carnot theorem, i.e., the Carnot efficiency being independent of the working substance. Therefore,
𝑄1𝑄2=𝑇1𝑇2
Q
2
Q
1
=
T
2
T
1
will always hold true for any working substance utilized in a Carnot engine.
Kinetic Theory Of Gases
The kinetic theory of gases is a model that explains the behavior of gases based on the motion of their particles. According to this theory:
-
Gases consist of tiny particles (atoms or molecules) that are in constant, random motion.
-
The volume of the particles themselves is negligible compared to the total volume of the gas.
-
The particles undergo elastic collisions with each other and with the walls of the container, transferring momentum but not losing energy.
-
The average kinetic energy of the gas particles is directly proportional to the temperature of the gas and is the same for all gases at the same temperature, regardless of their mass or chemical composition.
-
The pressure exerted by a gas is due to the collisions of its particles with the walls of the container.
Maxwell Law of Distribution of Molecules
Maxwell's law of distribution of molecules, also known as the Maxwell-Boltzmann distribution, describes the distribution of speeds (or velocities) of molecules in a gas at a particular temperature. It was developed by James Clerk Maxwell and Ludwig Boltzmann in the 19th century.
The key points of Maxwell's law of distribution are:
-
The distribution of molecular speeds in a gas follows a specific mathematical function known as the Maxwell-Boltzmann distribution curve.
-
According to this distribution, most molecules in a gas have speeds near the average speed, and there is a range of speeds extending in both directions from the average.
-
The distribution curve is bell-shaped, with the peak representing the most probable speed of molecules.
-
As temperature increases, the distribution curve spreads out, indicating that the range of molecular speeds becomes broader. This means that at higher temperatures, there is a greater proportion of molecules with higher speeds.
-
The distribution curve shifts to higher speeds as temperature increases, reflecting the fact that the average speed of molecules increases with temperature.
Benefits of CBSE Class 11 Physics Notes Chapter 12 Thermodynamics
CBSE Class 11 Physics Notes Chapter 12 on Thermodynamics provide several benefits for students:
Comprehensive Coverage:
These notes cover all the important concepts and topics related to thermodynamics as per the CBSE syllabus, providing students with a thorough understanding of the subject matter.
Simplified Explanation:
The notes present complex thermodynamic principles in a simplified manner, making it easier for students to grasp and comprehend the concepts.
Clarity and Organization:
The notes are well-organized, with clear explanations and examples provided for each topic. This helps students to follow along and understand the material more effectively.
Practice Questions:
Many notes include practice questions and problems, enabling students to test their understanding and reinforce their learning through practice.