
Cell Potential Formula: Cell Potential also known as electrode potential or electrochemical potential, is a fascinating concept that lies at the heart of electrochemistry. It is the driving force behind countless chemical reactions that power our modern world. In this article, we will explore the concept of cell potential, how it is calculated, and its real-world applications.
What Is Cell Potential Formula?
Cell potential is the measure of the potential difference between two electrodes in an electrochemical cell. It represents the ability of the cell to convert chemical energy into electrical energy. This concept is fundamental to our understanding of batteries, fuel cells, and many other electrochemical processes.
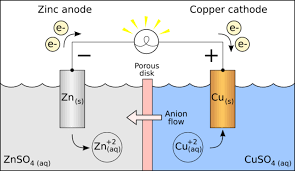
Cell Potential Chart
Imagine a map that guides you through the world of chemical reactions. The cell potential chart is precisely that – a visual representation of the cell potentials for various electrochemical cells. It provides crucial information about the feasibility and direction of chemical reactions. Each cell in the chart is associated with its half-reaction and standard cell potential.
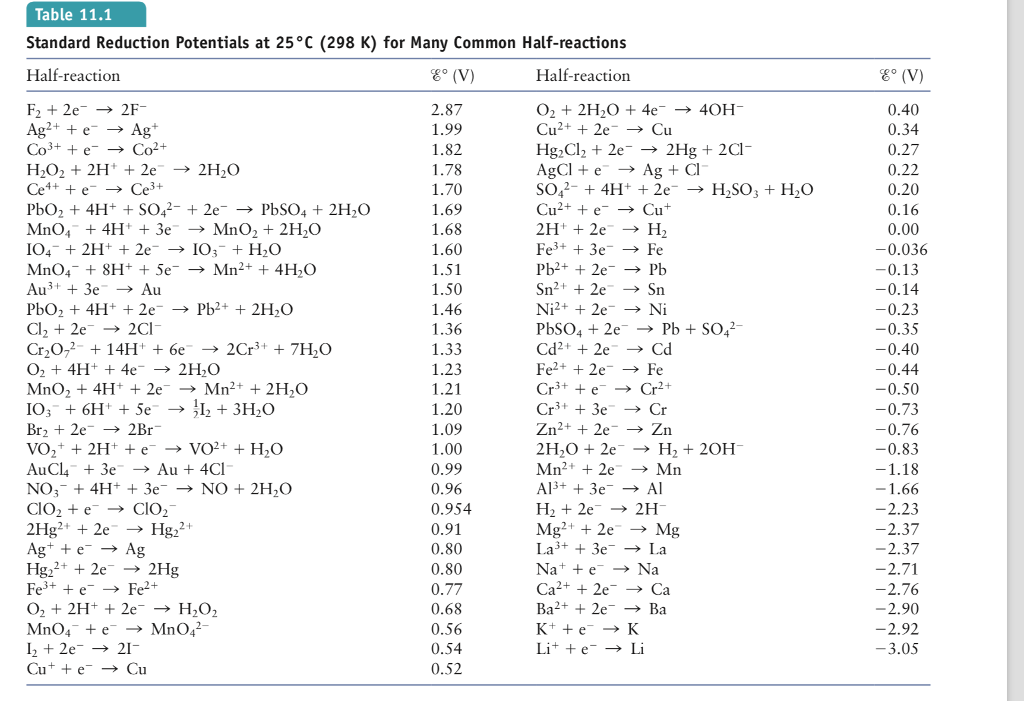
How to Find Cell Potential
Calculating cell potential involves understanding the underlying electrochemical reactions. The Nernst equation is a powerful tool that relates cell potential to the concentrations of reactants and products involved in the half-reactions. Here's a simplified step-by-step guide to finding cell potential:
- Identify the half-reactions occurring at the anode and cathode.
- Write balanced chemical equations for these half-reactions.
- Determine the standard cell potential (E°) for each half-reaction.
- Calculate the cell potential (E) using the Nernst equation.
Also Read – Kinematics Formula
Free Energy Calculation Of Cell Potential Formula
Gibbs free energy change (ΔG) is intimately connected to cell potential. This relationship allows us to determine whether a chemical reaction is spontaneous or non-spontaneous. The formula ΔG = -nFE relates ΔG (Gibbs free energy change), n (moles of electrons), F (Faraday's constant), and E (cell potential). A negative ΔG indicates a spontaneous reaction.
How to Calculate Electrochemical Potential
The electrochemical potential is the energy available per unit charge within an electrochemical cell. It is a fundamental concept in understanding the flow of electrons and ions in chemical reactions. The electrochemical potential (μ) can be calculated using the following formula:
μ = μ 0 + RT ln(Q)
Where:
- μ is the electrochemical potential.
- μ0 is the standard electrochemical potential.
- R is the gas constant.
- T is the temperature in Kelvin.
- Q is the reaction quotient.
Also Read – Rotatory Motion Formula
Electrochemical Potential Examples
To bring these concepts to life, let's explore some real-world examples of electrochemical potential calculations. Consider a galvanic cell with a copper electrode (Cu) and a zinc electrode (Zn). The half-reaction at the copper electrode is Cu²⁺(aq) + 2e⁻ → Cu(s), and at the zinc electrode, it's Zn²⁺(aq) + 2e⁻ → Zn(s). Using the Nernst equation and standard electrode potentials, we can calculate the cell potential for this cell and gain insights into the driving forces behind the reactions.
Solved Examples
Example 1: Calculating Cell Potential for a Galvanic Cell
Consider a galvanic cell where a copper electrode (Cu) is immersed in a 0.1 M Cu²⁺(aq) solution, and a zinc electrode (Zn) is immersed in a 0.01 M Zn²⁺(aq) solution. The half-reaction at the copper electrode is Cu²⁺(aq) + 2e⁻ → Cu(s), and at the zinc electrode, it's Zn²⁺(aq) + 2e⁻ → Zn(s).
- Identify the half-reactions:
Copper electrode: Cu²⁺(aq) + 2e⁻ → Cu(s)
Zinc electrode: Zn²⁺(aq) + 2e⁻ → Zn(s)
- Write balanced chemical equations:
Cu²⁺(aq) + 2e⁻ → Cu(s)
Zn²⁺(aq) + 2e⁻ → Zn(s)
- Determine standard cell potentials (E°) from tables:
E° for Cu²⁺/Cu is +0.34 V (cathode).
E° for Zn²⁺/Zn is -0.76 V (anode).
- Calculate cell potential (E) using the Nernst equation:
E = E°(cathode) - E°(anode)
E = (+0.34 V) - (-0.76 V)
E = +1.10 V
The cell potential for this galvanic cell is +1.10 V.
Also Read – Friction Formula
Example 2: Predicting Spontaneity
Let's determine if a reaction is spontaneous based on cell potential. Consider a cell with a standard cell potential (E°) of +0.20 V. We want to find out if the following reaction is spontaneous:
2 Fe³⁺(aq) + 3 Zn(s) → 2 Fe(s) + 3 Zn²⁺(aq)
- Write the half-reactions:
Oxidation (anode): 3 Zn(s) → 3 Zn²⁺(aq) + 6e⁻
Reduction (cathode): 2 Fe³⁺(aq) + 6e⁻ → 2 Fe(s)
- Calculate the cell potential (E) using the Nernst equation:
E = E°(cathode) - E°(anode)
E = (0 V) - (-0.76 V)
E = +0.76 V
Since the calculated cell potential (+0.76 V) is greater than zero, the reaction is spontaneous in the direction written. The cell potential confirms that the reaction will proceed spontaneously, with electrons flowing from zinc to iron.
These examples demonstrate how to calculate cell potential and determine the spontaneity of a reaction using electrochemical principles.
Lesson Summary
In summary, cell potential is a crucial concept in electrochemistry, representing the energy transformation in chemical reactions. It allows us to understand and predict the behavior of electrochemical cells, from batteries to fuel cells. By utilizing the Nernst equation and connecting cell potential to Gibbs free energy change, we can delve into the world of electrochemical spontaneity. Calculating electrochemical potential further enriches our understanding, revealing the energy within molecules. Through real-world examples, we've seen how these principles apply in practice.
In conclusion, cell potential is not just a concept confined to the laboratory; it's a fundamental force that shapes our modern world, enabling technological advancements and sustainable energy solutions.
Cell Potential Formula FAQs
What is cell potential, and why is it important in electrochemistry?
How is cell potential measured and expressed?
What is the Nernst equation, and how is it used to calculate cell potential?
How does cell potential relate to Gibbs free energy change (ΔG)?
What factors can influence cell potential in practical applications?










