
Raoults Law
Liquid Solution of Class 12
Raoult's Law
Vapour pressures of a number of binary solution of volatile liquids such as benzene and toluene at constant temperature gave the following generalization which is known as the Raoult’s law.
The partial pressure of any volatile component of a solution at any temperature is equal to the vapour pressure of the pure component multiplied by the mole fraction of that component in the solution.
Suppose a binary solution contains nA moles of a volatile liquid A and nB moles of a volatile liquid B, if PA and PB are partial pressure of the two liquid components, the according to Raoult’s law
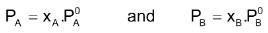
Where XA is the mole fraction of the component A given by nA/nA+ nB; xB is the mole fraction of the component B, given by nB/nA+nB and
 are the vapour pressures of pure components A and B respectively.
are the vapour pressures of pure components A and B respectively.
If the vapour behaves like an ideal gas, then according to Dalton’s law of partial pressures, the total pressure P is given by
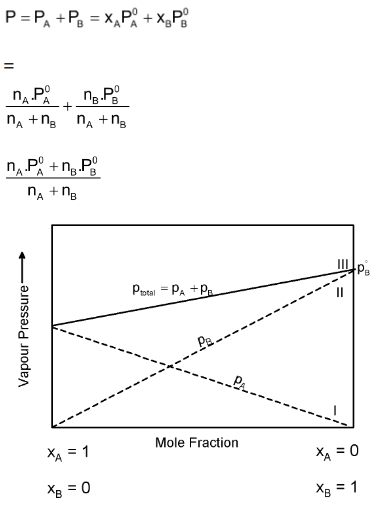
The relationship between vapours pressure and mole fraction of an ideal solution at constant temperature. The dashed lines I and II represent the partial pressure of the components. (It can be seen from the plot that pA and pB are directly proportional to xA and xB respectively). The total vapour pressure is given by line marked III in the figure.
Measurement of Vapour Pressure Lowering (Ostwald and Walker’s Apparatus)
In this method a stream of dry air is bubbled successively through (i) the solution (ii) the pure solvent and (iii) a reagent which can absorb the vapour of the solvent. As the solvent is usually water the reagent is generally anhydrous Calcium Chloride. The complete assembly is shown in the figure.
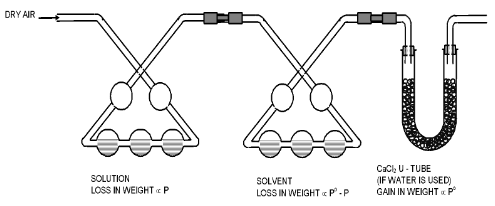
The first three bulbs contain a weighed amount of the solution under examination and the next three bulbs contain a weighed amount of the pure solvent. A weighed amount of anhydrous calcium chloride is taken in the set of U-tubes at the end.
All the bulbs must be kept at the same temperature and air must be bubbled gradually to ensure that it gets saturated with the vapours in each bulb.
The dry air, as it passes through the solution, takes up an amount of vapour which is proportional to the vapour pressure of the solution at the prevailing temperature. This moist air passes through water (solvent), it takes up a further amount of vapour which is proportional to the difference in vapour pressure of the pure solvent and the solution.
It is evident that,
Loss in mass of solution ∝ P
Loss in mass of solvent ∝ Po − P
Loss in mass of solution + loss in mass of solvent
∝ Po − P + P ∝ Po
Where P° = Vapour pressure of pure solvent, P = Vapour pressure of solution
The calcium chloride tubes are weighed at the end of the experiment. The gain in mass should be equal to the total loss in mass of the solution and solvent which, in turn, is proportional to P0, as shown above.
In other words,
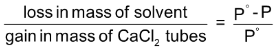
Thus, knowing the loss in mass of the solvent and gain in the mass of the calcium chloride tubes, it is possible to calculate the lowering of vapour pressure.









