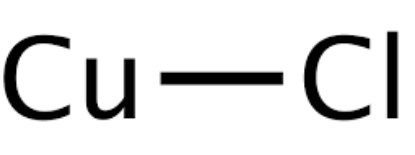Copper I Chloride Formula: Copper(I) chloride is also known as cuprous chloride. It is the lower copper chloride compound .CuCl. This material takes the form of a white solid, with limited solubility in water but high solubility in concentrated hydrochloric acid. When impurities are present, it may exhibit a greenish tint, typically attributed to the presence of copper(II) chloride (CuCl 2 ).
Copper, symbolized as Cu and occupying the 29th position on the periodic table, is a soft element renowned for its exceptional thermal and electrical conductivity. It displays a distinctive pinkish-orange hue. Chlorine (Cl), an element with an atomic number of 17 on the periodic table, exists as a greenish-yellow gas, exuding a pungent odor. This gaseous element poses harm to the environment and is 2-5 times denser than air. Among the halogens, it ranks as the second lightest element, boasting two stable isotopes.
Copper I Chloride Formula
Copper I Chloride Formula CuCl. It goes by various names, including Cuprous chloride, Copper monochloride, or Dicopper dichloride. This inorganic compound represents the lower chloride of copper and presents itself as a white crystalline solid. It may appear slightly green in color, owing to the presence of oxidation-induced impurities. Remarkably, it demonstrates insolubility in water and only rarely dissolves in concentrated hydrochloric acid.
Copper(I) chloride possesses a cubic zincblende crystal structure, but when subjected to heat, it undergoes a transformation into a hexagonal shape at around 408°C. This compound serves as a Lewis acid, characterized by the presence of vacant orbitals, with copper displaying a +1 oxidation state. This property enables it to readily accept electron pairs from Lewis bases.
Preparation of Copper I Chloride
Copper I Chloride Formula CuCl. The process for making Copper(I) Chloride involves two main methods:
By directly combining copper and chlorine at temperatures between 450 and 900°C:
2Cu + Cl 2 →2CuCl
Alternatively, it can be prepared by reacting Cuprite with Hydrogen Chloride:
Cu 2 O + 2HCl →2CuCl + H 2 O
Copper I Chloride Formula Physical Properties
Copper I Chloride Formula CuCl.
Molecular weight: It has a molecular weight of approximately 98.996 g/mol.
Density: The density of Copper(I) Chloride is about 4.14 g/cm³.
Boiling Point: It has a boiling point of 1,490°C.
Melting Point: The melting point of Copper(I) Chloride is 423°C.
Solubility in water: It exhibits limited solubility in water, approximately 0.047 g/L.
Copper I Chloride Formula Chemical Properties
Chemical Characteristics of Copper(I) Chloride
Copper(I) chloride exhibits intriguing chemical properties:
Dissolution in Halide Solutions: In aqueous solutions featuring halide ions, Copper(I) chloride dissolves and forms complexes. For instance, it can participate in the creation of species like H 3 O + and CuCl 2 - when combined with concentrated hydrochloric acid.
Complex Formation with Carbon Monoxide: When Copper(I) chloride is dissolved in solutions of HCl or NH3, it has the ability to absorb carbon monoxide, leading to the generation of colorless complexes.
Reaction with Oxygen: In the presence of oxygen and water, Copper(I) chloride can undergo a chemical reaction to produce Cu 3 Cl 2 (OH) 4 and CuCl 2 .
Uses of Copper I Chloride
Copper(I) Chloride Finds Diverse Applications:
It serves as a precursor in the manufacture of the fungicide copper oxychloride.
Copper(I) Chloride acts as a catalyst in various organic reactions.
Copper(I) Chloride plays a role in the production of silicone polymers, ethylene-propylene rubbers, dialkyl carbonates, and acrylonitrile.
In the petroleum industry, it functions as a desulfurizing agent.
It is used in the denitration of cellulose.
In the manufacturing of soaps, fats, and oils, it serves as a condensing agent.
When combined with carbon monoxide, hydrogen chloride, and aluminum chloride, it contributes to the synthesis of benzaldehydes.
It is utilized in the production of various other copper compounds.
Copper(I) chloride, also known as cuprous chloride, is an essential inorganic compound with a white solid appearance and distinctive properties. Its limited solubility in water and unique chemical characteristics make it valuable in a range of applications, from catalysis to the synthesis of various chemical compounds.
| Related Links | |
| Hydrobromic Acid Formula | Barium Iodide Formula |
| Barium Oxide Formula | Hydrogen Sulfate Formula |
Copper I Chloride Formula FAQs
What is Copper(I) chloride?
What is the appearance of Copper(I) chloride ?
Copper(I) chloride is soluble in water?
In what other solvent is Copper(I) chloride soluble?
What is the chemical formula for Copper(I) chloride?