
Copper Sulfate Formula: Copper(II) sulfate, also known as copper sulfate, is an inorganic chemical compound represented by the formula CuSO 4 . It has the ability to form hydrates with the formula CuSO 4 ·nH 2 O, where 'n' can vary from 1 to 7. The pentahydrate (when n = 5), which presents as a vibrant blue crystal ,It is the most frequently encountered form of hydrated copper(II) sulfate. This pentahydrate has been referred to by names such as blue vitriol, bluestone, vitriol of copper, and Roman vitriol.
When it dissolves in water, it releases heat and forms the aquo complex [Cu(H 2 O) 6 ] 2+ , characterized by an octahedral molecular structure. The solid pentahydrate exhibits a polymeric arrangement in which copper maintains its octahedral geometry but is bonded to four water ligands. These Cu(II)(H 2 O) 4 centers are linked by sulfate anions to create interconnected chains. On the other hand, anhydrous copper sulfate is a powdery substance with a light grey color.
Copper Sulfate Formula
Copper Sulfate Formula is CuSO 4. Copper sulfate can be referred to as either cuprous sulfate (Cu 2 SO 4 ) or cupric sulfate (CuSO 4 ). However, the term "copper sulfate" typically denotes CuSO 4 . This compound is scientifically known as copper(II) sulfate and has various common names such as blue vitriol, Roman vitriol, copper vitriol, and bluestone.
Copper Sulfate Formula Structure
The pentahydrate is the most frequently encountered form of copper sulfate, characterized by the chemical formula CuSO 4 ·5H 2 O and its distinctive bright blue color. It's noteworthy that the anhydrous variation of this compound presents itself as a white powder. The structure of CuSO 4 consists of an ionic bond between the copper cation (Cu 2+ ) and the sulfate anion (SO 4 2- ).
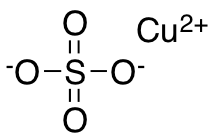
Copper Sulfate Formula Physical Properties
Copper Sulfate Formula is CuSO 4. The anhydrous copper sulfate has a molar mass of 159.609 grams per mole, while the pentahydrate version exhibits a molar mass of 249.685 grams per mole.
Anhydrous CuSO 4 presents as a grey-white, powdery substance. It is in contrast to the vivid blue appearance of the pentahydrate form.
The densities of anhydrous and pentahydrate CuSO 4 are 3.6 g/cm3 and 2.286 g/cm3.
Both the hydrated and anhydrous copper sulfates experience decomposition upon heating, leading to indistinct boiling points.
CuSO 4 ·5H 2 O crystals exhibit orthorhombic crystal structures, while anhydrous CuSO 4 crystals have triclinic structures.
Copper Sulfate Formula Chemical Properties
Copper Sulfate Formula is CuSO 4. Copper ions in copper sulfate react with chloride ions in concentrated hydrochloric acid, leading to the formation of tetrachlorocuprate (II), as described by the chemical equation:
Cu 2+ + 4Cl – → CuCl 4 2-
When heated to 650ºC, CuSO 4 undergoes a decomposition reaction, producing cupric oxide (CuO) and sulfur trioxide (SO 3 ).
Copper sulfate demonstrates high solubility in water, with solubility values of 1.055 molal at 10ºC and 1.502 molal at 30ºC.
Uses of Copper Sulfate
Copper sulfate, particularly in its pentahydrate form (CuSO 4 ·5H 2 O), finds various useful applications:
Due to its ability to combat a wide range of fungi, CuSO 4 .5H 2 O is used as a fungicide.
Copper sulfate is a key component in solutions like Benedict's and Fehling's, which are vital for sugar reduction tests.
It is used in the analysis of blood samples to diagnose conditions such as anemia.
In the process of vegetable dyeing, copper sulfate acts as a fixative for dyes.
Copper sulfate solutions in water are utilized in liquid resistors as resistive elements.
It can provide color to materials like cement, ceramics, and other metals, making it valuable for decorative purposes.
Copper sulfate is added to bookbinding glues to repel insects from printed paper, aiding in preservation.
Industrial Utilization
Copper sulfate finds extensive applications in various industries:
Copper Plating: It is used in the copper plating process.
Mining Industry: Copper sulfate serves as an activator in the concentration of lead, gold, and cobalt ores through froth flotation, proving beneficial to the mining sector.
Printing Industry: Its uses extend to the printing industry, functioning as an electrolyte in electrolytes production and an etching agent in print engraving.
Glass Coloration: Copper sulfate is significant for coloring glass.
Anti-Fouling Coatings: It's utilized in anti-fouling coatings to prevent the attachment of organisms to surfaces.
In essence, copper sulfate plays a versatile role across a wide spectrum of industries.
| Related Links | |
| Arsenic Acid Formula | Strontium Sulfate Formula |
| Strontium Chloride Formula | Barium Bromide Formula |
Copper Sulfate Formula FAQs
What is the chemical formula for copper sulfate?
Are there different forms of copper sulfate?
What distinguishes anhydrous copper sulfate from the pentahydrate form?
What are the common uses of copper sulfate?
Is copper sulfate used in the mining industry?










