
CBSE Worksheet for 4 Structure Of Atom class 9
Worksheet For class 9
Find CBSE Worksheet for chapter-4 Structure Of Atom class 9
CLASS-9
BOARD: CBSE
Chemistry Worksheet - 4
TOPIC: Structure Of Atom
For other CBSE Worksheet for class 9 Science check out main page of Physics Wallah.
STRUCTURE OF ATOM
SUMMAR Y –
- Discovery of electron, proton.
- Thompson’s model of atom.
- Rutherford’s model of atom and its defects.
- Discovery of neutron.
- Comparison of charge and mass of electron, proton and neutron.
- Bohr’s model of atom.
- Atomic number, mass number and electronic configuration.
-
Valency, isotopes, isobars, isotones, isoelectronic.
SECTION – 1
-
Which of the following statement is true ?
- A proton is 1837 times heavier than an electron.
- A proton is 1/1837 times heavier than an electron.
- A proton is 1837 times lighter than an electron.
- Proton has the same mass as an electron.
-
A proton is usually represented as –
-
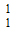
-
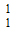 H
H
-
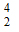 He
He
- both A) and B)
-
-
When alpha particles are sent through a thin metal foil , most of them go straight through the foil because –
- Alpha particles are much heavier than electron.
- Alpha particles are positively charged.
- Most part of the atom is empty.
- Alpha particles move with high velocity.
-
A neutron is represented as –
-
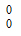 n
n
-
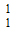 n
n
-
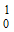 n
n
-
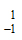 n
n
-
-
Many elements have non-integral masses because –
- They have isobars.
- Their isotopes have non-integral masses.
- They have isotopes.
- The constituents neutrons, protons and electrons combine to give fractional masses.
-
Two atoms of the same element are found to have different number of neutrons in their nuclei. These two atoms are –
- Isomers
- isotopes
- isobars
- allotropes
-
An atom which has a mass number of 14 and 8 neutrons is an –
- isotope of oxygen
- isobar of oxygen
- isotope of carbon
- isobar of carbon
-
The mass of proton is –
- 1.00728amu
- 1.673 × 10 -24 gm
- 1.673 × 10 -27 Kg
-
All of these
SECTION -2
-
From what observation do you derive the following inferences ?
- The most of the space inside the atom is empty.
- The volume of the nucleus is very small.
- Ar (40) and Ca (40) have the same mass number but their properties are entirely different. Why ?
- Define atomic number. Why is it called the main characteristic property of the elements ?
- Who proposed the existence of neutron and what was the basis of this assumption ?
- What type of information is obtained about the atom by Rutherford particle scattering experiment ?
-
- What are isotopes ? Give one example.
- Give the application of isotopes in industry and in medicine
-
The relative mass of an element A is 16.2. There are two isotopes
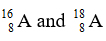 of the element.
of the element.
-
Calculate the percentage of these two isotopes present in the element.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.








