
NCERT Solutions for Class 10 Science Chapter 1: Excellent study materials are required for students studying in Class 10 CBSE Chemistry Chapter 1 Chemical Reactions and Equations, according to NCERT Solutions for Class 10 Science. Subject matter specialists have created these NCERT Solutions in accordance with the most recent CBSE syllabus.
It is crucial that students use NCERT Solutions to assist them learn how to solve and study so they can become familiar with the kinds of questions that are posed in the chapter as well as chemical reactions and equations.NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations Overview
NCERT Solutions for Class 10 Science Chapter 1 "Chemical Reactions and Equations," introduces students to the fundamental concepts of chemical reactions and how they are represented using chemical equations. The chapter explains the types of chemical reactions, such as combination, decomposition, displacement, and double displacement reactions. It also covers the concepts of oxidation and reduction (redox reactions).
NCERT Solutions for Class 10 Science Chapter 1 emphasizes the importance of balancing chemical equations to adhere to the law of conservation of mass. Students learn to write and balance equations for various reactions, including precipitation reactions and exothermic and endothermic reactions.
NCERT Solutions for Class 10 Science Chapter 1 Exercises
Here we have provided NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations -In-text questions set 1 Page number – 6
1. Why should a magnesium ribbon be cleaned before burning in the air?
Solution:
Because magnesium metal combines with ambient oxygen to generate magnesium oxide (MgO) layer, a very stable chemical, magnesium ribbon should be cleaned before burning in the air. Hence, the ribbon must be cleaned by removing the coating of MgO in order to stop more reactions with oxygen.2. Write a balanced equation for the following chemical reactions.
i) Hydrogen + Chloride —-> Hydrogen chloride
ii) Barium chloride + Aluminium sulphate —-> Barium sulphate + Aluminium chloride
iii) Sodium + Water —-> Sodium hydroxide + Hydrogen
Solution:
i) H 2 + Cl 2 → 2HCl ii) 3BaCl 2 + Al 2 (SO 4 ) 3 →3BaSO 4 + 2AlCl 3 iii) 2Na + 2H 2 O → 2NaOH + H 23. Write a balanced chemical equation with state symbols for the following reactions
i) Solutions of Barium chloride and Sodium sulphate in water react to give insoluble Barium sulphate and solution of Sodium chloride.
ii) Sodium hydroxide solution in water reacts with the hydrochloric acid solution to produce Sodium chloride solution and water.
Solution:
i) BaCl 2 + Na 2 SO 4 → BaSO 4 + 2NaCl ii) NaOH + HCl → NaCl + H 2 OIn-text questions set 2 Page number – 10
1. A solution of a substance, ‘X,’ is used for whitewashing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Solution:
i) The substance ‘X’ which is used in whitewashing is quick lime or Calcium Oxide and its formula is CaO . ii) CaO + H 2 O → Ca(OH) 22. Why is the amount of gas collected in one of the test tubes in Activity 1.7 double the amount collected in the other? Name this gas.
Solution:
In activity 1.7, water hydrolyses to release H2 and O2 gas, which results in twice as much gas being collected in one test tube as in the other. Here, two molecules of hydrogen and one molecule of oxygen gas are released during electrolysis; as a result, twice as much hydrogen would be recovered as oxygen.In-text questions set 3 Page number – 13
1. Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Solution:
Because iron is more reactive than copper, iron displaces copper from the copper sulphate solution when an iron nail is dipped in it. As a result, the copper sulphate solution's hue changes. The response is Fe + CuSO 4 → FeSO 4 + Cu2. Give an example of a double displacement reaction other than the one given in Activity 1.10.
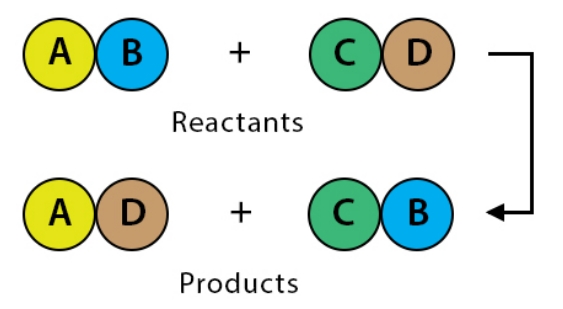
Solution:
3. Identify the substances that are oxidised and that are reduced in the following equation.
i) 4Na(s) + O 2 (g) → 2Na 2 O(s)
ii) CuO(s) + H 2 (g) → Cu(s) + H 2 O(l)
Solution:
The Sodium (Na) in the first equation is getting oxidized with the addition of Oxygen (O 2 ), and the Copper (Cu) in the second equation is reduced due to the addition of Hydrogen (H 2 ).Exercise Questions Page number – 14-16
1. Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb(s) + CO 2 (g)(a) Lead is getting reduced
(b) Carbon Dioxide is getting oxidised
(c) Carbon is getting oxidised
(d) Lead oxide is getting reduced
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all the above
Solution:
(i) (a) and (b) Explanation: (a) Because Oxygen is being removed and (b) Because the removed oxygen from Lead is added to the elemental Carbon.2. Fe 2 O 3 + 2Al → Al 2 O 3 + 2Fe
The above reaction is an example of a
- Combination reaction
- Double displacement reaction
- Decomposition reaction
- Displacement reaction
Solution:
The answer is 4. Displacement reaction. Aluminium oxide is created when oxygen from ferrous oxide is transferred to aluminium metal. Aluminium is a more reactive metal than Fe in this reaction. Al will so remove Fe from its oxide. One element displaces another in this kind of chemical reaction, known as a displacement reaction. In this instance, more reactive metal replaces less reactive metal. It is referred to as a single displacement reaction since it is a one-time displacement.3. What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.
- Hydrogen gas and Iron chloride are produced.
- Chlorine gas and Iron hydroxide are produced.
- No reaction takes place.
- Iron salt and water are produced.
Solution:
- Hydrogen gas and Iron chloride are produced.
4. What is a balanced chemical equation? Why should a chemical equation be balanced?
Solution:
When the number of distinct atoms on the reactant and product sides of the equation is equal, the equation is said to be balanced. Chemical equations must be balanced in order for the reaction to follow the Law of Conservation of Mass. There is no set procedure for balancing the chemical equation; it is just a matter of trial and error.5. Translate the following statements into chemical equations and balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in the air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give Aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and Hydrogen gas.
Solution:
(a) Unbalanced: H 2 + N 2 → NH 3 Balanced: 3H 2 + N 2 → 2NH 36. Balance the following chemical equations.
(a) HNO 3 + Ca(OH) 2 → Ca(NO 3 ) 2 + H 2 O
(b) NaOH + H 2 SO 4 → Na 2 SO 4 + H 2 O
(c) NaCl + AgNO 3 → AgCl + NaNO 3
(d) BaCl 2 + H 2 SO 4 → BaSO 4 + HCl
Solution:
(a) 2HNO 3 + Ca(OH) 2 → Ca(NO 3 ) 2 + 2H 2 O7. Write the balanced chemical equation for the following reactions.
Calcium hydroxide + Carbon dioxide —-> Calcium carbonate + Water
Zinc + Silver nitrate —-> Zinc nitrate + Silver
Aluminium + Copper chloride —-> Aluminium chloride + Copper
Barium chloride + Potassium sulphate —-> Barium sulphate + Potassium chloride
Solution:
2Ca(OH) 2 + 2CO 2 → 2CaCO 3 + 2H 2 O Zn + 2AgNO 3 → Zn(NO 3 ) 2 + 2Ag 2Al + 3CuCl 2 → 2AlCl 3 + 3Cu BaCl 2 + K 2 SO 4 → BaSO 4 + 2KCl8. Write a balanced chemical equation for the following and identify the type of reaction of each case.
KBr + BaI 2 → KI + BaBr 2
ZnCO 3 → ZnO + CO 2
H 2 + Cl → HCl
Mg + HCl → MgCl 2 + H 2
Solution:
2KBr + BaI 2 → 2KI + BaBr 2 (Double Displacement Reaction) ZnCO 3 → ZnO + CO 2 (Decomposition Reaction) H 2 + Cl → 2HCl (Combination Reaction) Mg + 2HCl → MgCl 2 + H 2 (Displacement Reaction)9. What is meant by exothermic and endothermic reactions? Give examples.
Solution:
When energy is taken up from the environment in the form of heat (such as during photosynthesis, ice melting, or evaporation), an endothermic process takes place. On the other hand, an exothermic reaction is one in which energy is released into the environment from the system (Examples: nuclear fission and fusion, concrete setting, explosions).10. Why is respiration considered to be an exothermic reaction?
Solution:
Energy is necessary for life to survive. Food is the source of this energy for us. During the digestive process, food molecules are broken down into simpler molecules, such as glucose. When these materials interact with the oxygen found in our body's cells, carbon dioxide, water, and a little quantity of energy are produced (the respiration process). As the energy used to sustain our body temperature takes the form of heat, respiration is regarded as an exothermic reaction. The response that is occurring is: C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + Energy11. Why are decomposition reactions called the opposite of Combination reactions? Write equations for decomposition reactions.
Solution:
The decomposition process is defined as the splitting of bigger molecules into two or more smaller molecules, whereas the combination reaction is defined as the reaction of two or more molecules to generate a larger molecule. This basically clarifies how the decomposition process differs from the combination reaction. Since the heat from the environment or heat-induced breaks the bonds of the bigger molecule, the breakdown mechanism is usually endothermic. Several instances of breakdown reactions include ZnCO 3 → ZnO + CO 2 CaCO 3 + Energy → CaO + CO 2 2HgO → 2Hg + O 212. Write one equation each for decomposition reactions in which energy is supplied in the form of heat, light or electricity.
Solution:
(a) Thermal decomposition reaction (Thermolysis) Decomposition of potassium chlorate: When heated strongly, potassium chlorate decomposes into potassium chloride and oxygen. This reaction is used for the preparation of oxygen. 2KClO 3 + Heat → 2KCl + 3O 2 (b) Electrolytic decomposition reaction (Electrolysis) Decomposition of sodium chloride: On passing electricity through molten sodium chloride, it decomposes into sodium and chlorine.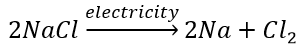 (c) Photodecomposition reaction (Photolysis) Decomposition of hydrogen peroxide: In the presence of light, hydrogen peroxide decomposes into water and oxygen.
(c) Photodecomposition reaction (Photolysis) Decomposition of hydrogen peroxide: In the presence of light, hydrogen peroxide decomposes into water and oxygen. 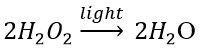
13. What is the difference between displacement and double displacement reactions? Write relevant equations for the above.
Solution:
A double displacement reaction occurs when two compounds exchange ions, while a displacement reaction occurs when a more reactive substance removes a less reactive one from its salt solution. A displacement reaction involves only one displacement between the molecules, whereas a double displacement reaction involves two displacements, as the name implies. Example: Displacement reaction Mg + 2HCl → MgCl 2 + H 2 Double displacement reaction 2KBr + BaI 2 → 2KI + BaBr 214. In the refining of Silver, the recovery of silver from Silver nitrate solution involves displacement reaction by Copper metal. Write down the reaction involved.
Solution:
Cu(s) + 2AgNO 3 (aq) → Cu(NO 3 ) 2 (aq) + 2Ag(s)15. What do you mean by a precipitation reaction? Explain by giving examples.
Solution:
Ions are exchanged between the compounds in a double displacement reaction that occurs when two solutions containing soluble salts are mixed. One of these compounds sinks to the bottom of the container as it solidifies and becomes insoluble in water.16. Explain the following in terms of the gain of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Solution:
(a) In a chemical reaction, when the oxygen is added to the element to form its respective oxide it is the element being oxidised. Example: 4Na(s) + O 2 (g) → 2Na 2 O(s) H 2 S + O 2 → H 2 O + SO 2 (b) In a chemical reaction, when the oxygen is removed from the compound, then it is said to be reduced. Example: CuO(s) + H 2 (g) → Cu(s) + H 2 O(l) 2HgO → 2Hg + O 217. A shiny brown coloured element ‘X’ on heating in the air becomes black in colour. Name the element ‘X’ and the black-coloured compound formed.
Solution:
The shiny brown-coloured element is Copper metal (Cu). When the metal is heated in air, it reacts with atmospheric oxygen to form copper oxide. Hence, the black-coloured compound is copper oxide. 2Cu(s) + O 2 (g) → 2CuO(s)18) Why do we apply paint on iron articles?
Solution:
Paint is used on iron objects to stop them from rusting. If the metal surface is not coated, it will come into contact with airborne oxygen and, in the event of moisture, iron(III) oxide. However, painting shields the surface from air and moisture, which stops rusting.19) Oil and Fat containing food items are flushed with Nitrogen. Why?
Solution:
The major goal of flushing nitrogen into food packages containing fat and/or oil is to stop rancidity, which happens when the fat or oil combines with oxygen to release an off-putting taste and smell. Consequently, an unreactive environment is produced by flushing nitrogen, preventing rancidity.20) Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Solution:
(A) The process of corrosion involves oxidising refined metal with air to create oxides, which are more stable compounds. When the metal corrodes, it steadily deteriorates. One example of corrosion in which iron is transformed into iron oxide is the rusting of iron. Every year, millions of dollars are spent to keep monuments like bridges from rusting. (B) The situation that results in an unpleasant taste and smell due to the aerial oxidation of the fat and oil in the food item. Food stored in the refrigerator slows down its rancidity because the low temperature inhibits the oxidation process.NCERT Solutions for Class 10 Science Chapter 1 PDF Download
NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations provides a strong foundation for understanding chemical processes and prepares students for more advanced topics in chemistry. Here we have provided science class 10 chapter 1 question answer pdf download for the ease of studentsNCERT Solutions for Class 10 Science Chapter 1
Study without using the internet
Benefits of NCERT Solutions for Class 10 Science Chapter 1
NCERT Solutions for Class 10 Science Chapter 1, "Chemical Reactions and Equations," offer several benefits to students:
Clarity of Concepts : NCERT Solutions for Class 10 Science Chapter 1 provide detailed explanations for each concept, helping students understand the types of chemical reactions, the significance of balancing equations, and the underlying principles of chemical processes.
Step-by-Step Problem Solving : NCERT Solutions for Class 10 Science Chapter 1 break down complex problems into manageable steps, guiding students through the process of writing and balancing chemical equations, which is essential for mastering the topic.
Practice and Revision : With a variety of solved examples and exercises, students can practice different types of problems, reinforcing their learning and improving their problem-solving skills.
Exam Preparation : NCERT Solutions for Class 10 Science Chapter 1 are aligned with the NCERT curriculum and exam pattern, helping students prepare effectively for board exams by focusing on important topics and frequently asked questions.
Self-Assessment : NCERT Solutions for Class 10 Science Chapter 1 enable students to assess their understanding and identify areas where they need further practice, allowing for targeted revision and better exam performance.
NCERT Solutions for Class 10 Science Chapter 1 FAQs
What are NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations?
How can NCERT Solutions for Chapter 1 help in exam preparation?
Are NCERT Solutions for Class 10 Science Chapter 1 sufficient for understanding chemical reactions?
Do NCERT Solutions for Chapter 1 include explanations for balancing chemical equations?
What types of chemical reactions are covered in NCERT Solutions for Chapter 1?










