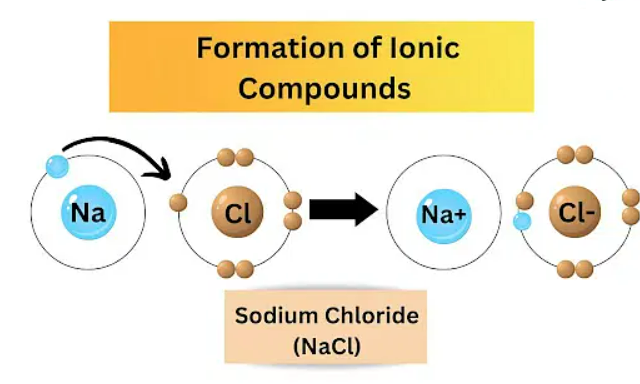
Cations and anions comprise ionic compounds; all ionic compounds contain a specific ionic unit with a particular charge. The amount of cation and anion in an ionic compound is always the same. In their chemical equation, ionic compounds reflect their ionic units and their ratio of cations to anions.
Ionic compound formula
There is nothing complicated about writing an ionic compound formula. Simply write the cation symbol followed by the anion symbol. Then, find the lowest multiple of the ions to neutralize the compound. Even though the ionic compound consists of charged ions, its formula and overall composition are electrically neutral.
- A cation's total positive charge equals an anion's total negative charge
- Usually, ionic compounds consist of a combination of metals and nonmetals. These guidelines ensure uniformity in writing ionic compound formulas.
- An ion is always placed before a cation.
Also Read - Cobalt II Nitrate Formula
Ionic Compound Formation
It is interesting to see what happens when metals and non-metals react. An ionic bond occurs when an electron transfers from a metal to a non-metal. An ionic bond is an electrostatic force of attraction between two atoms with opposite charges. An ionic bond occurs when an electron transfers from a metal to a non-metal. Cations and anions form ionic bonds. Anions have a negative charge, and cations have a positive charge.
What is an Ionic Compound?
An atom's number of protons, neutrons, and electrons makes it unique. Atoms are usually equal in number to the number of protons and electrons. Protons in an atom cannot change because that would mean having a completely different element. However, electrons can change in an atom.
An atom can get an ion when it gains or loses electrons. Since electrons already have a net negative charge, their charge changes when added or removed. As a result, the number of electrons is no longer in sync with the positive charge of protons.
Also Check - Malic Acid formula
As a result of gaining an electron, anions get a net negative charge. In contrast, cations get a net positive charge when atoms lose electrons and obtain a net negative charge. Anions are usually nonmetals, whereas cations are usually metals. Also, ions can refer to a single atom or multiple complex groups of atoms.
Opposites indeed attract when it comes to ions. The bonding of opposite charges, negative and positive forms ionic compounds. As its name suggests, it is a compound made up of ions. One atom's loss or gain matches the other's loss or gain. A pair is formed when the atom donates an electron to the atom that needs it.
Whenever you try to put the south end and the north end of two different magnets together, they repel each other. When you turn one magnet around, the north end is positioned to a sound end, which causes magnets to snap together instantly. A common salt is an ionic compound combining positive and negative ions. Ionic compounds include sodium chloride, also known as common salt.
Cation - A cation has fewer electrons than protons, resulting in a net positive charge. To form a cation, an element must lose one or more electrons to another element that has a strong affinity for them. A ‘+’ sign denotes a cation. Example: Magnesium2+
Anion - In an anion, there are more electrons than protons, resulting in a net negative charge. An anion is formed when an element gains one or more electrons that another element with a weaker affinity for those electrons might otherwise lose. The '-' sign indicates a cation, such as oxygen.
Also Check - Potassium Cyanide formula
Properties of Ionic Compound
- Physical Properties - Positive and negative ions are attracted towards each other, which makes ionic compounds solid and not easily breakable. However, they break into pieces when pressure is applied, making them brittle.
- Melting and Boiling Point - As ionic compounds have a strong force of attraction, it takes a lot of energy to break the ionic bonds between the atoms, which is why they have a higher melting and boiling point.
- Solubility - As well as polar solvents such as water, methanol, and formamide, ionic compounds are insoluble or barely soluble in non-polar solvents such as chloroform, hydrocarbons, etc.
- Electric Conductivity - Unlike solid compounds, ionic compounds conduct electricity when molten. The conduction of electricity involves the transfer of charge from one place to another.
- Since ions cannot move in solid state, ionic compounds can't conduct electricity. However, molten ionic compounds can conduct electricity since their electrostatic forces of attraction are overcome by heat.
- They are brittle.
Structure of Ionic Compound
Ionic compounds, such as salts, oxides, sulfides, and hydroxides (as well as many inorganic substances), depend on the size of their cations and anions to ensure they form adequately. For instance, when sodium and chloride ions attract one another, forming a 3-D structure, we get none other than a sodium chloride crystal. The total charge of this structure is zero because the number of positive ions is equivalent to the number of negative ones—all thanks to the electrostatic forces that keep these ions together.
Ionic character formula
It determines the difference in electronegativity between two atoms in polar covalent bonds.
Δχ = χB − χA
The difference in electronegativity increases with bond polarity and ionic character.
Example of an Ionic Compound
When Magnesium reacts with chlorine
By reacting with chlorine, magnesium loses two electrons from its outermost orbit, i.e., two electrons from the M shell are removed and the L shell becomes the outermost orbit. As a result, it forms a stable octet. With 12 protons and only 10 electrons, Mg has a positive charge.
Similarly, in the case of chlorine, the outermost orbit has seven electrons. Therefore, Cl needs one electron to complete a stable octet. The Mg ion gives Cl one electron. However, Mg released two electrons while Cl only needed one electron, so the two Cl atoms combined to form a negative charge Cl. In this reaction, MgCl2 is formed.
Applications Of Ionic Compounds
- NaCl (Sodium Chloride) can be found in everyday tablets
- NaF (Sodium Fluoride) is a common ingredient in toothpaste.
- NaHCO₃ (Sodium bicarbonate), also known as baking soda, is widely used in cooking and baking.
- On the other hand, Mg(OH)₂ (Magnesium hydroxide) is an antacid that helps calm acidity.
- K₃PO₂ (Potassium phosphate) acts as a food additive.
- MgSo₄ (magnesium sulfate) is employed to purify drinking water.
- Ionic compounds also used for wood preservation, such as boric acid and oxides; they may also function as growth regulators like gibberellic acid.
- In addition, due to their resistance to organic solvents, they are ideal for paint pigmentation.
Ionic Compound Formula FAQs
What is an ionic compound?
How are ionic compound formulas determined?
What is the formula of sodium chloride (table salt)?
How are polyatomic ions represented in ionic compound formulas?
Can the charges on ions vary in ionic compounds?