
Aluminium formula, symbolized by "Al" on the periodic table, is an essential element that plays a significant role in various aspects of science and industry. Besides being silvery-white, aluminum is also soft, ductile, and non-magnetic in nature. It accounts for approximately 8% of the Earth's crust. Furthermore, it is the third most abundant element and the most abundant metal in the Earth. Alumina's occurrence decreases below the mantle of the Earth. Also, bauxite is its primary ore.
Aluminium Formula
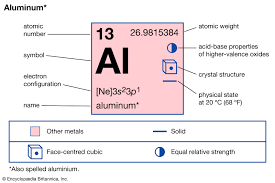
The aluminium formula is simply "Al." As an element, it exists in its pure form, comprising individual aluminium atoms. It is the most abundant metal in the Earth's crust, making up around 8% of its solid surface. The name "aluminium" is derived from the Latin word "alumen," which means "bitter salt." Sir Humphry Davy, an English chemist, first isolated aluminium in the early 19th century. In some regions, it is spelt as "aluminium" by the International Union of Pure and Applied Chemistry (IUPAC) naming convention.
Aluminum Formula Structure
In its pure form, aluminium is a metallic element with a face-centred cubic (fcc) crystal structure. This arrangement allows aluminium to exhibit its characteristic metallic properties, such as malleability, ductility, and electrical conductivity.
Aluminum Formula and Valency
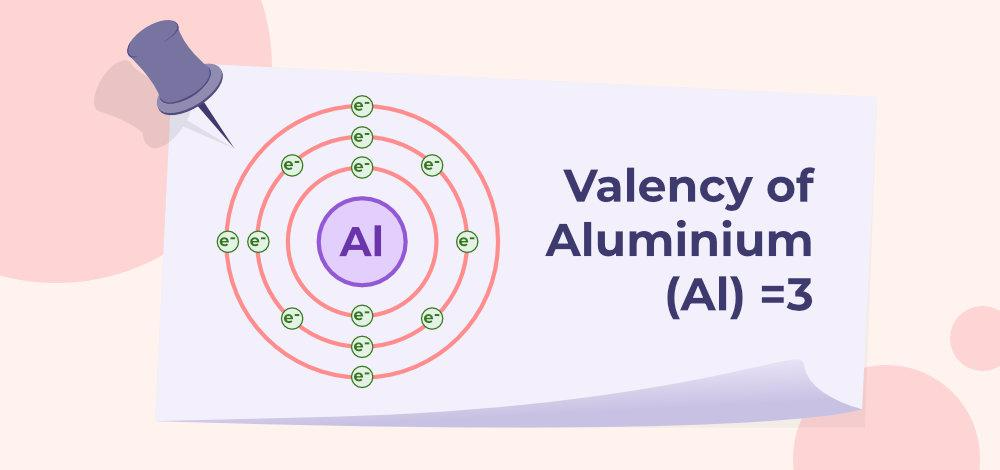
Aluminium formula typically exhibits a valency of +3. This means that chemical compounds tend to lose three electrons to achieve a stable electron configuration, forming Al³⁺ ions. This valency plays a crucial role in forming various aluminium compounds and the bonding it undergoes with other elements.
Aluminum Formula Mass
The atomic mass of the aluminium formula is approximately 26.98 atomic mass units (u) or 26.98 g/mol (grams per mole). This value represents the average mass of aluminium atoms, considering the different isotopes of aluminium found in nature.
Aluminium Formula in Nature
Aluminium formula is highly abundant in nature and is the most prevalent metal in the Earth's crust. It occurs primarily in the form of bauxite ore, which is a mixture of aluminium hydroxides and other minerals. Extracting aluminium from bauxite is a key industrial process, as aluminium is widely used in aerospace and construction industries.
Aluminium Formula Ion
The ion of aluminium is Al³⁺. This ion is formed when an aluminium atom loses its three valence electrons to achieve a stable electron configuration. The Al³⁺ ion plays a critical role in the formation of ionic compounds and the overall chemistry of aluminium.
Also Read : Camphor FormulaAluminum Formula of Oxide
Aluminium oxide, also known as alumina, has the chemical formula Al₂O₃. It comprises two aluminium ions (Al³⁺) and three oxygen ions (O²⁻). Aluminium oxide is a widely used compound in various applications, including as a refractory material, abrasive, and as the primary component of rubies and sapphires, giving them their brilliant red and blue colours.
Electronic Configuration of Aluminum
Furthermore, if the amount of electrons and protons is equal, then the element becomes neutral. This is because the positives and negatives tend to cancel one another out.
Additionally, one can find the atomic number and atomic mass of aluminium on the periodic table of elements. Aluminium is also a lightweight metal with a silvery appearance.
A silver ion has a charge of +3. This is because the aluminium atom has three electrons in its valence. For the noble gas to satisfy the octet rule, three electrons must be removed. The ion will thus gain a positive charge of 3 when it loses three electrons.
A molecule of aluminium weighs 26.98 grams. It is a metallic element with a 2.7 grams per cubic centimetre density. Its atomic number is 13. Its atom has 13 protons, 14 neutrons, and 13 electrons.
Also Read : Chlorine Gas FormulaPhysical Properties of Aluminium
The silver or light grey powder of aluminium is easily ignited. Further, when aluminium burns, it emits an intense flame. Aluminum uncoated powder resembles a light grey solid. It is also denser than water. Aluminium can burn mucous membranes, eyes, and skin if it comes into contact with them. In addition to being toxic, it can also be inhaled or ingested if it comes in contact with them.
By nature, aluminium is odourless. Furthermore, aluminium has a boiling point of 4221 °F/2327 °C at 760 mm Hg and a melting point of 1220 °F/660 °C. Moreover, this element is insoluble in organic solvents and water. However, dilute hydrochloric acid, hot water, and alkaloids dissolve it.
Also Read : Copper FormulaChemical Properties of Aluminium
Aluminium has many characteristics, including its small size and highly charged nature. Al3+ is a small and highly charged cation, and several aluminium compounds tend toward covalency. In contrast to other post-transition metals, aluminium's valence shell is based on the noble gas that precedes it. Consequently, aluminium does not suffer from incomplete valence electrons, unlike the other post-transition metals.
Moreover, aluminium has a highly negative standard electrode potential, similar to lanthanum, actinium, yttrium, and scandium, which contain ds2 configurations of three valence electrons.
Aluminium reacts with most non-metals when heated, forming compounds such as aluminium sulfide, aluminium nitride, and aluminium halides. Aluminium is also capable of forming a wide range of intermetallic compounds. In addition, aluminium reacts with water with hydrogen evolution in the presence of hot, concentrated hydrochloric acid.
| Related Links | |
| Formaldehyde Formula | Glucose Chemical Formula |
| Formic Acid Formula | Glutaric Acid Formula |
Aluminium Formula FAQs
Is aluminium Al or Al₂?
Is aluminium a symbol or formula?
Why is the formula Al₂O₃?
Why is aluminium called Al?










