
It is a bicyclic monoterpene ketone found in plants like Cinnamomum Camphora, with a chemical formula of C 10 H 16 O. Kampfer is a colorless to white waxy crystalline powder with a strong scent similar to mothballs, and it can be flammable. Extracted from the camphor laurel wood, Camphor Oil has various properties, including anti-inflammatory and analgesic, acting as an insect repellant, and being used in topical skin preparations and embalming fluids.
Camphor Formula Structure
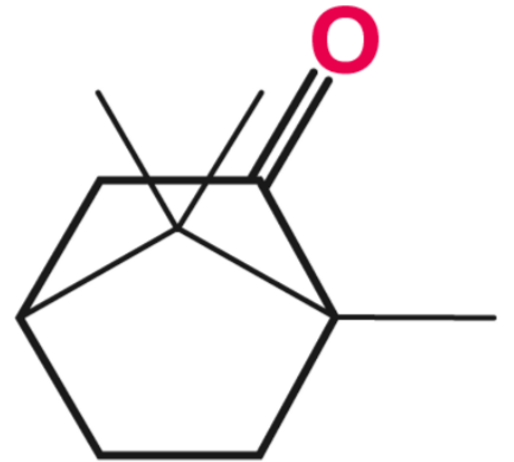
In the above image, camphor is identified as a monoterpene ketone with an oxygen substituent at the 2nd carbon, connected by a double bond. Being a bicyclic compound, the carbons where both rings attach are referred to as bridgehead carbons, held together by covalent bonds known as bridgebonds or bridgeheadbonds. These same bridgehead carbons also serve as chiral centers for camphor, giving it optical activity. It can exist in four distinct stereoisomeric forms: (+)-camphor, (-)-camphor, (+)-isoborneol, and (-)-isoborneol.
What is Camphor?
It is a bicyclic monoterpene ketone compound found in plants like Cinnamomum Camphora. It is a colorless to white waxy crystalline powder. It has a strong aroma like that of a mothball. Its oil comes from camphor laurel wood. Its properties include anti-inflammatory, analgesic, insect repellent, and is used in various topical skin preparations and embalming fluids.
This compound is found in the camphor laurel, and is quite common in China, Taiwan, and Japan. The vapors can be condensed by passing steam through the pulverized wood. In order to purify it, the oily portion of the distillate must be crystallized and sublimated. Camphor belongs to the terpenoid ketones group of organic compounds. It melts between 178 and 179 degrees Celsius.
Also Check – Theoretical Yield Formula
Types of Camphor
Below are the different types of camphor
Natural camphor - It is obtained naturally from plants that produce camphor, particularly Cinamommum camphora. It has been marketed for many years as a forest product.
Synthetic camphor - It is derived from turpentine oil, specifically from the alpha-pinene found in coniferous tree oils. Acetic acid is used as a solvent and strong acid as a catalyst to convert alpha-pinene into isobornyl acetate. Hydrolysis of isobornyl acetate yields isoborneol, which is then oxidized to produce racemic camphor.
Also Check – Dilution Formula
Production of Camphor
A biosynthetic method can be used to produce camphor using geranyl pyrophosphate. Geranyl pyrophosphate is converted to linaloyl pyrophosphate, which is further converted to bornyl pyrophosphate. Bornyl pyrophosphate is then hydrolyzed into borneol, which is then oxidized to form camphor.
Through the mevalonic acid pathway, geranyl pyrophosphate is converted to bornyl pyrophosphate. The reaction occurs in cells, so the reaction conditions are not well defined, but the enzyme bornyl diphosphate synthase is required to convert geranyl pyrophosphate to bornyl pyrophosphate. A series of steps are then followed by the oxidation of borneol or isoborneol into camphor to convert bornyl pyrophosphate into camphor enzymatically.
In order to produce camphor via chemical synthesis, borneol or isoborneol must undergo oxidation. This process involves dissolving the borneol in a mixture of acetone and ethanol, which is then acidified with chromic acid in sulphuric acid. The resulting solution is heated and refluxed for several hours to initiate the reaction. After cooling, any excess acid is removed using reducing agents. Finally, the isolated camphor can be obtained by dissolving it in non-polar solvents and evaporating the solvents.
Also Check – Degree Of Unsaturation Formula
Uses
It is widely used as an antipruritic, anti-infective agent, mothproofing agent, flavoring agent, and pharmaceutical agent. The bark and wood of the camphor tree are distilled to make this camphor, which is usually made from turpentine oil. It is commonly found in Vicks VapoRub, for example.
In addition to rubbing on the skin, camphor products can also be inhaled. People use camphor to relieve pain, reduce itching, and reduce the urge to cough. There is good evidence supporting these uses. In addition to treating toenail fungus, warts, insect bites, cold sores, and hemorrhoids, Camphor can also be applied to the skin. There is, however, no good scientific research to back these other uses up.
Camphor should never be applied to broken skin since it can enter the body quickly and reach toxic concentrations.
Camphor Formula FAQs
Q1. What is the chemical formula of camphor?
Q2. What is the common use of camphor?
Q3. Is camphor safe for ingestion?
Q4. What is the source of natural camphor?
Q5. Can camphor be used to repel insects?










