
Copper II Chloride Formula: Copper(II) chloride, which is also referred to as cupric chloride. It is an inorganic substance characterized by the chemical formula CuCl 2 . The anhydrous variant of this compound, presenting a monoclinic structure and a yellowish-brown color. Its gradually absorbs moisture to give rise to the dihydrate form, which is orthorhombic and blue-green in appearance and is denoted as CuCl 2 ·2H 2 O due to its association with two water molecules.
This compound is primarily produced on an industrial scale and serves as a co-catalyst in the Wacker process. Its anhydrous and dihydrate forms can be found in nature, though they are quite rare. In their natural state, they occur as the minerals tolbachite and eriochalcite, respectively.
Copper II Chloride Formula Structure
Copper II Chloride Formula is CuCl 2 . Copper(II) chloride, or cupric chloride, is an inorganic compound . It can exist in two forms: a monoclinic yellowish-brown anhydrous form that gradually absorbs moisture, transforming into an orthorhombic blue-green dihydrate CuCl 2 ·2H 2 O, which contains two water molecules of hydration.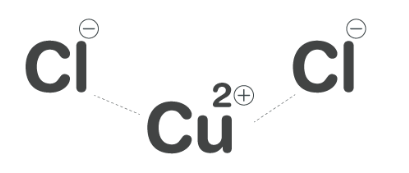
Copper II Chloride Formula Physical Properties
Copper II Chloride Formula is CuCl 2 .Here are some key physical properties of Copper(II) Chloride:The molecular weight of Copper(II) Chloride is 134.45 g/mol.
It has a melting point of 498°C.
The boiling point of Copper(II) Chloride is 993°C.
The density of CuCl2 is 3.386 g/cm3.
Copper II Chloride Formula Chemical Properties
Copper II Chloride Formula is CuCl 2 . Copper(II) Chloride exhibits corrosive properties when in contact with aluminum.
Regarding its oxidation state, Copper(II) Chloride is characterized by a metal oxidation state of +2. It serves as a relatively mild oxidizing agent and, when it interacts with aluminum foil, it generates hydrogen, Copper(I) oxide, and aluminum chloride.
Upon heating and CuCl 2 reacts with NaOH (sodium hydroxide), it results in the formation of sodium(II) hydroxide while liberating chlorine gas.
Preparation of Copper II Chloride
Copper II Chloride Formula is CuCl 2 . Commercially, Copper(II) chloride is produced through a process involving the chlorination of copper. This procedure entails heating copper to a red-hot temperature, typically in the range of 300-400°C, and allowing it to react directly with chlorine gas. The result is the production of molten copper(II) chloride, and this reaction is notably highly exothermic.
The chemical equation representing this reaction is as follows:
Cu(s) + Cl 2 (g) → CuCl 2 (l)
To obtain a solution of copper(II) chloride, a different method is used in commercial production. Chlorine gas is introduced into a circulating mixture of hydrochloric acid and copper. From this solution, the dihydrate form of copper(II) chloride can be obtained through the process of evaporation.
Uses Copper II Chloride
Copper II Chloride Formula is CuCl 2 . Copper(II) Chloride finds a wide range of applications in various fields:
It is used as a wood preservative, fungicide, insecticide, and herbicide.
In the field of laundry, Copper(II) Chloride is utilized in marking inks.
This compound plays a crucial role in the manufacturing of agricultural chemicals.
It is used in water treatment processes.
In the petroleum industry, Copper(II) Chloride serves as a deodorizing agent, helping to eliminate offensive odors.
It functions as an oxidizing and purifying agent.
In the textile industry, Cupric dichloride acts as a mordant for dyeing and printing textiles.
In both organic and inorganic reactions, CuCl 2 serves as a catalyst. Notably, it acts as a catalyst in the production of chlorine from HCl.
Natural Occurrence
Copper(II) chloride is a rare find in nature, occurring naturally in two distinct forms. The anhydrous mineral, tolbachite, and the dihydrate, eriochalcite. These are both exceptionally uncommon. These minerals are typically discovered in proximity to fumaroles and can occasionally be found in certain copper mines.
More commonly, mixed oxyhydroxide-chlorides such as atacamite (Cu 2 (OH) 3 Cl) are found. These are often associated with oxidation zones in copper ore deposits, especially in arid climates.Safety and Biological Impact
Copper(II) chloride can pose health risks and environmental concerns:
Copper(II) chloride has the potential to be harmful. The US Environmental Protection Agency permits only concentrations of aqueous copper ions in drinking water that are below 1.3 ppm. When copper chloride is absorbed, it can lead to symptoms such as headaches, diarrhea, lowered blood pressure, and fever. Ingesting significant quantities may result in copper poisoning, central nervous system disorders, and haemolysis.
Copper(II) chloride is considered an environmental pollutant. It is often present in irrigation-grade water and can have adverse effects on water and soil microbes. Denitrifying bacteria, in particular, are highly sensitive to the presence of copper(II) chloride. At a concentration of 0.95 mg/L, it was found to inhibit the metabolic activity of denitrifying microbes by 50% (IC50).
| Related Links | |
| Hydrobromic Acid Formula | Barium Iodide Formula |
| Barium Oxide Formula | Hydrogen Sulfate Formula |
Copper II Chloride Formula FAQs
What is Copper(II) Chloride?
What are the main uses of Copper(II) Chloride?
How is Copper(II) Chloride commercially prepared?
Is Copper(II) Chloride exothermic during its formation?
What is the molecular weight of Copper(II) Chloride?










