
Lead IV Oxide Formula: Lead(IV) oxide, also known as anhydrous Plumbic acid, lead dioxide, or Plumbic oxide, has the chemical formula PbO 2 . In this compound, the oxidation state of lead is +4. It serves as a potent oxidizing agent. Notably, Plumbic oxide does not dissolve in alcohol or water, remaining insoluble in these liquids. This substance presents as a dark brown, glass-like powder and finds widespread applications in the production of electrodes, explosives, and matchsticks. It can dissolve in various acids such as hydrochloric acid, nitric acid, and oxalic acid. PbO 2 is also utilized in the manufacturing process of rubber substitutes.
Lead IV Oxide Formula
Lead(IV) oxide, with the formula PbO 2 , is a compound where lead has an oxidation state of +4. It is used as an oxidizing agent in explosives and dye production, a curing agent for polysulfide, an anode material in electrochemistry, and in various other applications, including preventing copper corrosion, manufacturing lead-acid storage batteries, and producing rubber substitutes. It also plays a role in lightning arrester production.
Lead IV Oxide Formula Structure
Lead IV oxide, with an accurate mass and monoisotopic mass of 239.966 g/mol, exhibits a unique structure. In its structure, it features two hydrogen bond acceptors and no hydrogen bond donors. This compound consists of one covalently bonded unit and is considered canonicalized.
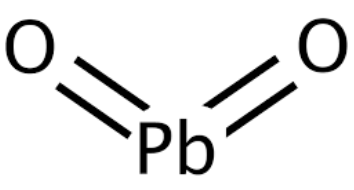
Lead IV Oxide Formula Physical Properties
Lead IV oxide exhibits the following physical properties:
Molar Mass: The molar mass of lead oxide is 239.1988 g/mol.
Appearance: It appears as a dark brown substance or in the form of a black powder.
Density: The density of lead oxide is 9.38 g/cm³.
Melting Point: It has a melting point of 290 °C (554 °F; 563 K).
Solubility: Lead IV oxide is insoluble in water and alcohol but is soluble in acidic acetic acid.
Lead IV Oxide Formula Chemical Properties
Let's explore some chemical reactions of lead IV oxide:Decomposition
When lead dioxide is heated in the presence of air, it decomposes as follows:24 PbO 2 → 2 Pb 12 O 19 + 5 O 2
Pb 12 O 19 → Pb 12 O 17 + O 2
2 Pb 12 O 17 → 8 Pb 3 O 4 + O 4
2 Pb 3 O 4 → 6 PbO + O 2
Reaction With Acids and Bases
Lead dioxide (PbO 2 ) is an amphoteric compound, exhibiting both acidic and basic properties. When it dissolves in a strong base, it forms the hydroxyl plumbate ion, [Pb(OH) 6 ] 2 − :PbO 2 + NaOH + 2 H 2 O → Na 2 [Pb(OH) 6 ]
Lead dioxide reacts with hot acids, where the Pb4+ cation is unstable and converts to a stable Pb 2+ state, releasing oxygen:2PbO 2 + 2H 2 SO 4 → 2PbSO 4 + 2H 2 O + O 2
2PbO 2 + 4HNO 3 → 2Pb(NO 3 ) 2 + 2H 2 O + O 2
PbO 2 + 4HCl → PbCl 2 + 2H 2 O + Cl 2
Oxidizing Agent
Lead dioxide is known for its strong oxidizing properties. It participates in various redox reactions as an oxidizing agent:MnSO 4 + 5 PbO 2 + 6 HNO 3 → 2 HMnO 4 + PbSO 4 + Pb(NO 3 ) 2 + 2 H 2 O
Cr(OH) 3 + 10 KOH + 3 PbO 2 → K 2 CrO 4 + K 2 PbO2 + 8 H 2 O
Production of Lead IV Oxide
Lead IV oxide can be produced by the reaction of Pb3O4 with nitric acid:Pb 3 O 4 + HNO 3 → PbO 2 + Pb(NO 3 ) 2 + H 2 O
Alternatively, plumbic oxide can be synthesized by treating lead chloride with sodium hypochlorite (NaClO) . The decomposition of lead(IV) oxide (PbO 2 ) can be represented by the following chemical equation:2 PbO 2 → 2 PbO + O 2
In this reaction, lead(IV) oxide breaks down into lead(II) oxide (PbO) and oxygen gas (O 2 ) .Applications of Lead IV Oxide
Lead IV Oxide finds a variety of uses in different industries, including:
- Manufacturing of Explosives
- Production of dyes as an oxidizing agent
- Utilized as a curing agent for polysulfide
- Anode material in electrochemistry
- Electrolyte for copper to prevent corrosion
- Utilized as an analytical reagent
- Important in the production of rubber substitutes
- Used in the manufacture of lightning arresters
- Material industries use lead oxide as an oxidizing agent
- Component in lead-acid storage batteries
| Related Link | |
| Potassium Acetate Formula | Porpionic Acid Formula |
| Phthalic Acid Formula | Pentane Formula |
Lead IV Oxide Formula FAQs
What is the chemical formula of Lead(IV) Oxide?
What is the oxidation state of lead in Lead(IV) Oxide?
Is Lead(IV) Oxide soluble in water and alcohol?
What is the melting point of Lead(IV) Oxide?
In what type of acids is Lead(IV) Oxide soluble?










