
Potassium Iodate Formula is KIO 3 ,Potassium is an important mineral that is required by all body tissues, and it is known as an electrolyte due to its role in carrying small electrical charges, which are responsible for activating various cell and nerve functions. Natural sources of potassium can be found in many foods and supplements.
Iodate, with the chemical formula IO -3 , is the predominant form of iodine in nature, primarily due to its presence in the primary iodine ore. Iodine salts, including iodate, are typically colorless.
Potassium Iodate Formula
When it comes to potassium iodate, it should be noted that it functions as an oxidizing agent. Therefore, contact with flammable materials or reducing agents can potentially lead to combustion. This compound consists of K + ions and IO 3– ions in a 1:1 ratio, making it an ionic compound.
Although potassium iodide is infrequently used, it is used in common salt iodide, offering a practical method for measuring iodine. In numerous countries, potassium iodide serves as a source of iodine and is even included in some infant formulas. Additionally, potassium iodate, much like potassium bromate, is utilized as a ripening agent for baked goods.
Potassium Iodate Formula and Structure
The potassium iodate formula is KIO 3 . It is synthesized by the reaction of potassium base with iodic acid. Potassium iodate is the primary compound used to iodize table salt, offering a practical method for iodine measurement. Additionally, it finds application in precipitating thorium, particularly for the removal of thorium from rare earth materials.
The structure of potassium iodate is characterized by its chemical formula, KIO 3 , with a molar mass of 214.001 g/mol. It consists of K + ions and IO 3– ions, encompassing potassium, iodide, and oxygen. The structure of potassium iodate is as follows:
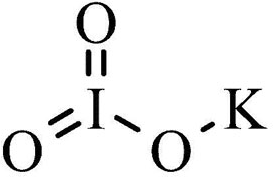
Potassium Iodate Formula Charge
The potassium iodate formula is KIO 3 . In this compound, the potassium ion (K + ) has a charge of +1, and the iodate ion (IO 3- ) has a charge of -1.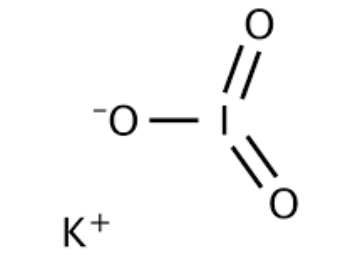
Potassium Iodate Formula Physical Properties
Potassium iodate is characterized by its lack of odor.
It consists of two covalent units.
It melts at 560°C, with partial decomposition releasing oxygen.
Its density is 3.90 g/cm3.
It has the capacity to accept three hydrogen bonds, serving as hydrogen bond acceptors.
It is soluble in potassium iodide (KI) solution.
Potassium iodate contains five heavy atoms.
Its molar mass is approximately 49.8 g/mol.
Potassium Iodate Formula Chemical Properties
When potassium iodate reacts with potassium iodide in the presence of strong acids like sulfuric acid, it forms potassium sulfate, iodine, and water:
KIO 3 + 5KI + 3H 2 SO 4 → 3K 2 SO 4 + 3H 2 O + 3I 2
Potassium iodate also reacts with silver nitrate to produce silver iodate and potassium nitrate:
KIO 3 + AgNO 3 → AgIO 3 + KNO 3
Preparation of Potassium Iodate
Potassium iodate, a substance with oxidizing properties, poses a fire risk when it comes into contact with flammable materials or reducing agents. It can be synthesized through various methods, such as the reaction of a potassium-containing base, like potassium hydroxide (KOH), with iodic acid, as shown in the following example:
HIO 3 + KOH → KIO 3 + H2O Another method involves adding iodine to a concentrated, heated potassium hydroxide solution: 3I 2 + 6KOH → KIO 3 + 5KI + 3H 2 O Alternatively, potassium iodide can be fused with substances like potassium chlorate, bromate, or perchlorate. The resulting melt is then treated with water, and potassium iodate can be separated from the solution through crystallization: KI + KClO 3 → KIO 3 + KClUses of Potassium Iodate
Potassium iodate is used in the iodization of common salt, where it can be oxidized to iodine when exposed to molecular oxygen under humid conditions.
It finds application in the food industry as a ripening agent and dough conditioner, contributing to food product quality.
Potassium iodate serves as a reagent in various chemical processes and is also added as a supplement in feed for specific purposes.
It plays a role in the analysis of samples containing arsenic and zinc.
Potassium iodate is utilized in iodometry, an analytical technique, and is a component in pharmaceutical manufacturing.
| Related Links | |
| Gold Formula | Phosphate Formula |
| Helium Formula | Hydrazine Formula |
Potassium Iodate Formula FAQs
What is the chemical formula of potassium iodate?
How is potassium iodate structured at the molecular level?
What is potassium iodate?
What are the charges of the ions in the potassium iodate formula?










