
Ammonium chloride formula , known as 'sal ammoniac', is a compound of ammonia and hydrogen chloride. It has various applications, such as providing nitrogen in fertilizers and serving as an electrolyte in dry cells. Additionally, it is commonly used in galvanizing, tinning, and soldering fluxes to eliminate oxide coatings from metals and enhance adhesion between solders. Its effectiveness as an expectorant also makes it a common ingredient in cold and cough remedies.
In addition to being crystalline and colourless, ammonium chloride is highly soluble when mixed with water. It is produced by the ammonia-soda process in forming sodium carbonate, which also produces ammonia chloride as a by-product. Alternatively, the reaction between ammonium sulfate and sodium chloride solutions can also produce ammonia gas. However, when ammonia chloride is mixed with calcium carbonate, it produces an ammonia gas.Ammonium Chloride Formula - Acid and Base
Ammonium chloride can act as both an acid and a base. In aqueous solutions, it can donate a proton (H+) to act as an acid or accept a proton to act as a base. This property is due to the compound's ammonium ion (NH4+).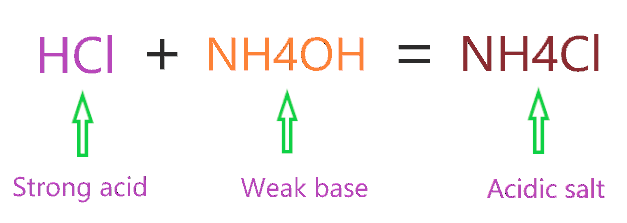
Also Read: Phthalic Acid Formula
Ammonium Chloride Formula by Crisscross Method
The formula of ammonium chloride is determined using the crisscross method, which involves swapping the subscripts of the elements in the compound's ions. In this case, the ammonium ion (NH4+) has a charge of +1, and the chloride ion (Cl-) has a charge of -1. To balance the charges, the formula becomes NH4Cl.Ammonium Chloride Formula Equation
The chemical equation for forming ammonium chloride typically involves the reaction of ammonia (NH3) and hydrochloric acid (HCl). The balanced equation is: NH3 + HCl → NH4Cl This equation represents the ammonia and hydrochloric acid combination to produce ammonium chloride.Ammonium Chloride Formula Structure
Ammonium chloride has a crystalline structure in its solid form. It consists of NH4+ and Cl- ions arranged in a lattice structure held together by ionic bonds. The ammonium ion is tetrahedral, with each hydrogen atom bonded to the central nitrogen atom.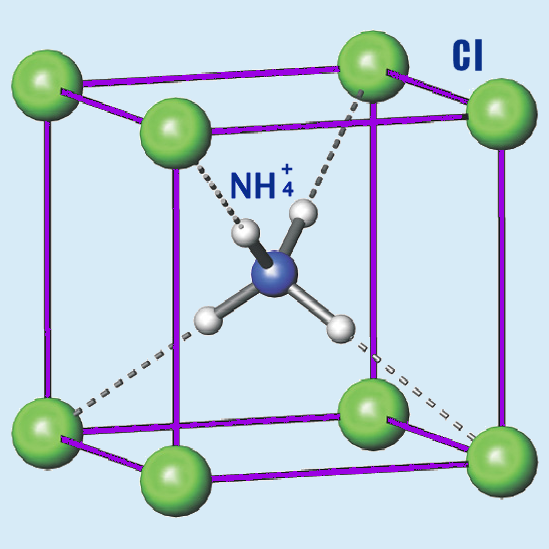
Also Read: Water Formula
Ammonium Chloride Formula Charge
The ammonium ion (NH4+) has a charge of +1, while the chloride ion (Cl-) has a charge of -1. When these ions combine to form ammonium chloride, the overall charge of the compound is neutral, as the +1 charge of the ammonium ion cancels out the -1 charge of the chloride ion.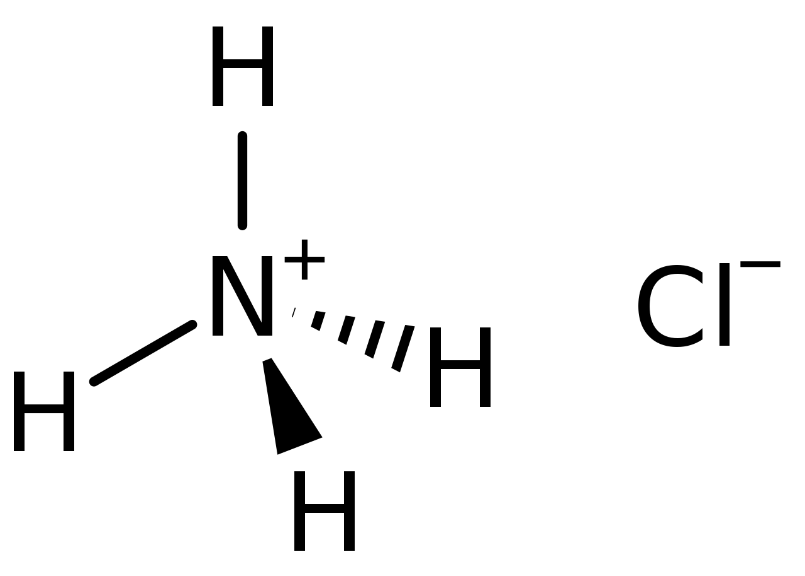
Ammonium Chloride Formula Balanced Equation
The balanced chemical equation for the reaction between ammonia and hydrochloric acid to form ammonium chloride has already been mentioned: NH3 + HCl → NH4Cl This equation shows that one molecule of ammonia reacts with one molecule of hydrochloric acid to produce one molecule of ammonium chloride.Also Read: Nickel Chloride Formula
Ammonium Chloride Formula Mass
To calculate the formula mass (molar mass) of ammonium chloride, you can add the atomic masses of the individual elements in the compound. The molar mass of NH4Cl is approximately 53.49 grams per mole, which is the sum of the atomic masses of one nitrogen atom (N), four hydrogen atoms (H), and one chlorine atom (Cl).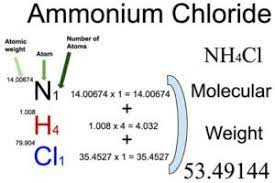
Uses of Ammonium Chloride Formula
Ammonium chloride has various practical uses. It can act as a flux when preparing metals for galvanization, soldering, and tin coating by cleaning the surface of workpieces and forming volatile metal chlorides. Due to its effectiveness, ammonium chloride is readily available in block form at hardware stores for easy use in cleaning soldering iron tips and as flux in solder. This versatile compound is also commonly used as an expectorant in cough medicines, where it irritates the bronchial mucosa to stimulate excessive fluid production and alleviate coughing. Additionally, ammonium salts can be used as gastric irritants to induce vomiting and nausea when needed. Its chemical formula is NH₃NO₃, and its boiling point is 56 degrees Celsius. Furthermore, aqueous solutions of ammonium chloride serve as electrolytes in Leclanche cells, commonly used in 'local batteries' installed in subscribers' telephones for commercial use.| Related Links | |
| Aspirin Formula | Azelaic Acid Formula |
| Glycerol Formula | Hexane Formula |
Ammonium Chloride Formula FAQs
What is NH4Cl called?
How is NH4Cl formed?
What is another name for ammonium chloride?
What is the valency factor of NH4Cl?










