
"Periodic Table Formula: classification organizes chemical elements based on increasing atomic number, revealing recurring patterns and properties, grouped into periods and families."
Periodic classification is based on blocks and also provides trends in various atomic properties.Blocks
s-block: Elements: Groups 1 and 2 (Alkali metals and Alkaline earth metals) General electronic configuration: ns 1-2 Where "n" is the outermost shell.
p-block: Elements: Groups 13 to 18 (Boron, Carbon, Nitrogen, Oxygen, Halogens, and Noble gases) General electronic configuration: ns 2 np 1-6 "n" can be the second or greater principal quantum number.
d-block: Elements: Transition metals (Groups 3 to 12) General electronic configuration: (n-1) d 1-10 ns 0-2 The "d" orbitals being filled belong to an inner shell compared to the "s" orbitals.
f-block: Elements: Lanthanides and Actinides General electronic configuration: (n-2) f 1-14 (n-1) d 0-1 ns 2 The "f" orbitals being filled are two shells inside compared to the "s" orbitals.
Ionization Energy (IE)
Definition: Ionization energy is the energy required to remove the most loosely bound electron from a neutral atom in its gaseous state. Equation: For a generic atom, A, the equation for the first ionization energy is given by:A (g) →A + (g) + e –
The energy for this process is the first ionization energy. If we talk about the second ionization energy, it would be for the removal of another electron from A + , and so on for subsequent ionization energies.Electron Affinity (EA)
Definition: Electron affinity is the energy change when a neutral atom in the gaseous state accepts an electron to form a negatively charged ion. Equation: For a generic atom, B, the equation for electron affinity can be represented as:B (g) + e − →B − (g)
The energy for this process indicates the electron affinity. Note that while electron affinity is usually exothermic (releases energy), it can also be endothermic (requires energy) for some elementsElectronegativity
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It plays a fundamental role in understanding the type and nature of chemical bonds between atoms. Various scales have been developed to quantify electronegativity.Also Check – Molar Volume formula
Pauling's Scale
Linus Pauling introduced this widely used scale. It's based on the bond energies of different compounds. Formula: The difference in electronegativity between two atoms, A and B, can be related to the bond energy of the A–B bond, E AB , by the following equation: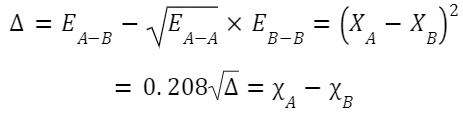 where E
AA
and E
BB
are the bond dissociation energies for the diatomic molecules A–A and B–B respectively.
Mulliken's Scale: Proposed by Robert S. Mulliken. This scale is based on the average of the ionization energy (IE) and the electron affinity (EA) of an atom. Formula:
where E
AA
and E
BB
are the bond dissociation energies for the diatomic molecules A–A and B–B respectively.
Mulliken's Scale: Proposed by Robert S. Mulliken. This scale is based on the average of the ionization energy (IE) and the electron affinity (EA) of an atom. Formula:
![]()
Electronegativity Difference Method: It's a simple method where the type of bond (ionic or covalent) is determined based on the difference in electronegativity values between the two bonded atoms. General Guidelines: If Δχ (difference in electronegativity) is less than 0.5, the bond is typically considered nonpolar covalent. If Δχ is between 0.5 and 1.7, the bond is polar covalent. If Δχ is greater than 1.7, the bond has significant ionic character.
Also Check – Aluminium Acetate Formula
Pauling Electronegativity Difference Method
Linus Pauling provided guidelines to determine the character of a bond based on the difference in electronegativity
(Δχ) between the two bonded atoms.Formula for Percent Ionic Character:
Also Check – Periodic Acid formula
Hannay-Smith Electronegativity Difference Method
Hannay and Smith proposed a method similar to Pauling's but with slightly different boundaries for bond types. Their method was based on observed dipole moments and theoretical ionic bond contributions. General Guidelines: If Δχ is less than 0.4, the bond is nonpolar covalent. If Δχ is between 0.4 and 1.4, the bond is polar covalent. If Δχ is greater than 1.4, the bond is ionic.Periodic Table Formula FAQs
Q1. Define electronegativity.
Q2. Why does atomic size generally decrease across a period?
Q3. How does metallic character vary across the periodic table?
Q4. Why do elements in the same group have similar chemical properties?
Q5. What is the difference between ionization energy and electron affinity?










