
Ammonium acetate is a white crystalline solid created when ammonia and acetic acid react and is also known as ammonium ethanoate or azanium acetate. Predominant uses of Ammonium acetate involves chemical analysis, such as within pharmaceutical and food processing industries, along with its application as a buffer substance in tropical personal care products like shampoos, skin lotions, and conditioners. The IUPAC name for ammonium acetate is CH3COONH4.
What is Ammonium Acetate?
It is a salt formed by combining acetic acid and ammonia. Its chemical name is Ammonium Acetate, also called the spirit of Mindererus in its watery form. This salt has various uses, from food preservation to medicines and chemical analysis. It is highly effective as an acidity regulator in food; however, it can be dangerous to the environment and all living creatures.
Also Read: Boltzmann Constant
Ammonium Acetate Structural Formula
As Ammonium Acetate salt consists of a weak acid and a weak base, it is often combined with acetic acid to make a buffer solution. Because it has been used to replace cell buffers with non-volatile salts in the preparation of chemical samples, ammonium acetate is volatile at low pressures. The chemical formula for ammonium acetate is as follows.
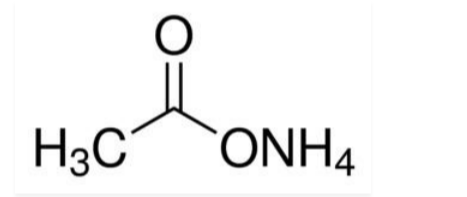
Ammonium Acetate Molecular Formula
Ammonium Acetate is the combination of a weak base and a weak acid. It is frequently used to prepare buffers because of its volatility when exposed to low temperatures. The molecular weight of ammonium acetate is approximately 77.083 g/mol, with a density ranging from 1.17 g/cm3 (20 °C) to 1.073 g/cm3 (25 °C). Its chemical formula is NH4CH3CO2.
Production of Ammonium acetate
Acetates can be made in the same way as other acetates, by neutralizing acetic acid. Furthermore, we employ acetic acid, which is used to neutralize ammonium carbonate in the process. In addition, in the chemical industry, this is produced by saturation of ammonia-glacial acetic acid:
Also Read - Acetone Formula
Ammonium Acetate Reactions
As an acetamide precursor, ammonium acetate acts as follows:
It's also used as a diuretic.
Why is Ammonium acetate Buffer?
Ammonium acetate is a salt formed by combining a weak acid and base, and is primarily utilized as an acetic acid to create a buffer solution. It can be produced by either neutralizing acetic acid with ammonium carbonate or saturating glacial acetic acid with ammonia. However, crystalline ammonium acetate production is difficult due to its hygroscopic nature. Additionally, it is often used in HPLC with ELSD detection by replacing cell buffers with non-volatile salts for sample preparation. Ammonium formate and other volatile salts are also used as buffer solutions for mobile phases.
Also Check - Actetamide Formula
Properties Of Ammonium Acetate Formula
| Chemical formula | NH4CH3CO2 |
| Molecular weight | 77.083 g/mol |
| Density | 1.17 g/cm3 (20 °C) 1.073 g/cm3 (25 °C) |
| Melting point | 113 °C (235 °F; 386 K) |
Uses of Ammonium Acetate
- Low temperatures make ammonium acetate unstable.
- The biodegradable de-icing agent is ammonium acetate.
- Explosives are commonly manufactured with ammonium acetate.
- Foam rubber is made from ammonium acetate.
- As a preservative and acidity regulator in food, ammonium acetate is used.
- Acetates of ammonium are unitized in agricultural products.
- Vinyl plastics are manufactured using ammonium acetate.
- Pharmaceutical industries rely heavily on components of ammonium acetates.
Health hazards of Ammonium Acetate
- If ammonium acetate dust is breathed in any way, it can irritate the nose and mouth.
- Swallowing this salt can cause rashes when it comes in contact with your lips and stomach.
- Rashes can occur if this chemical is exposed to the eyes.
- The same is true when ammonium acetate comes in contact with the skin. This substance might cause gastrointestinal and respiratory discomfort if exposed.
Ammonium Acetate Formula FAQs
What is the common use of ammonium acetate in chemistry?
Is ammonium acetate a hazardous chemical?
What is the molar mass of ammonium acetate?
Can ammonium acetate be used in food products?
Does ammonium acetate have any odor or smell?










