
Cadmium nitrate formula is Cd(NO3)2. This inorganic salt is composed of cadmium (Cd) cations and nitrate (NO3-) anions. Let's delve into the various aspects of cadmium nitrate, including its molecular weight, uses, and its hydrated forms. Cadmium nitrate formula, also known as Cadmium (II) nitrate formula or Cadmium dinitrate formula. The crystalline form of Cadmium nitrate is colourless and emits toxic cadmium oxide fumes when heated. Cd(NO3)2 is its molecular or chemical formula.
When exposed to this compound, it causes severe irritation to the skin, respiratory system, and eyes. It can also cause lung damage, shortness of breath, chest pain, and decrease renal function. It is a known carcinogen with an elevated risk of developing lung cancer.
Cadmium Nitrate Formula
It was Karl Samuel Leberecht Hermann and Friedrich Stromeyer who discovered and isolated cadmium for the first time in 1817. The elemental form of cadmium is silvery bluish-gray metallic in color. Inorganic, it is crystalline and colorless. It produces toxic fumes on heating. Cadmium nitrate formula is also referred to as Cadmium (II) nitrate or Cadmium dinitrate formula.
As a result of exposure to this compound, the skin, respiratory system, and eyes are severely irritated. Additionally, it damages the lungs, causing chest pain, shortness of breath, and kidney damage, which decreases renal function. It is a known carcinogen, which increases the risk of developing lung cancer.
It is generally possible to dissolve nitrate compounds in water and oxidise them. When mixed with hydrocarbons, nitrate compounds are normally flammable. Cadmium Nitrate is available immediately in most of the volumes and is ideal as a precursor to make ultra-high purity compounds, certain catalysts, and nanoscale materials. The high purity submicron and nanopowder forms may also be considered.
During the year 1817, Karl Samuel Leberecht Hermann and Friedrich Stromeyer discovered and isolated cadmium for the first time. The elemental form of cadmium is silvery bluish gray metallic in color. It produces toxic cadmium oxide fumes when heated. Cadmium nitrate is also known as Cadmium (II) nitrate or Cadmium dinitrate.
As a result of exposure, this compound causes severe irritation to the skin, respiratory tract, and eyes. As well as damaging the lungs, it can cause shortness of breath, chest pain, and kidney damage, resulting in decreased renal function. It is a known carcinogen that increases the risk of developing lung cancer.
Nitrate compounds are generally soluble in water. Nitrate compounds are also a kind of oxidizing agent. If mixed with hydrocarbons, nitrate compounds can normally produce flammable mixtures. Nitrates are excellent precursors for producing ultra-high purity compounds, catalysts, and nanoscale materials. Cadmium Nitrate formula is generally available immediately in most volumes and can be used in high purity submicron and nanopowder forms.
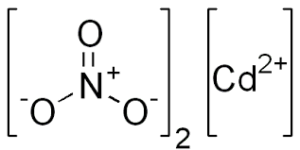
Also Read: Ammonium Sulfide Formula
Cadmium Nitrate Molecular Weight
The molecular weight of cadmium nitrate formula, calculated by adding the atomic weights of its constituent elements, is approximately 236.42 grams per mole. This value is important for various chemical calculations and determining the quantity of cadmium nitrate in a given sample.
Also Read: Barium Acetate Formula
Cadmium Nitrate Tetrahydrate
Cadmium nitrate can exist in hydrated forms. Cadmium nitrate tetrahydrate, with the chemical formula Cd(NO3)2·4H2O, is one of the most common hydrates of cadmium nitrate formula. The "tetrahydrate" designation indicates four water molecules for every cadmium nitrate unit.
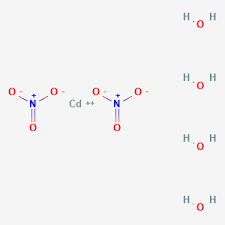
Cadmium Nitrate Tetrahydrate Molecular Weight
The molecular weight of cadmium nitrate tetrahydrate can be calculated by considering the weights of its constituent elements, including cadmium, nitrogen, oxygen, and hydrogen. It is approximately 308.44 grams per mole.
Cadmium Nitrate Solubility
Cadmium nitrate is highly soluble in water. This solubility is a critical property for its use in various applications, as it readily dissolves in aqueous solutions, making it convenient for processes like electroplating and chemical reactions.
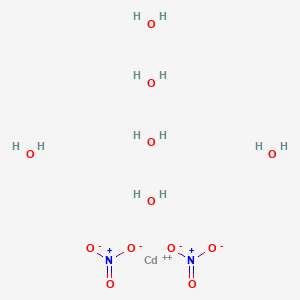
Cadmium Nitrate Hexahydrate
Cadmium nitrate can also exist in a hexahydrate form, denoted as Cd(NO3)2·6H2O. In this hydrated state, it contains six water molecules for every cadmium nitrate unit. This hexahydrate is less common compared to the tetrahydrate form but retains its water-soluble nature and some application versatility.
Properties Of Cadmium Nitrate Formula
Cadmium nitrate, with the chemical formula Cd(NO3)2, is an inorganic compound with several notable properties:
| Properties of Cadmium Nitrate Formula | |
| Chemical formula | Cd(NO 3 ) 2 |
| Molecular weight | 236,42 g/mol |
| Density | 3.6 g/cm 3 (anhydrous) 2.45 g/cm 3 (tetrahydrate) |
| Boiling point | 132 °C (tetrahydrate) |
| Melting point | 360 °C (anhydrous) 59.5 °C (tetrahydrate) |
Cadmium Nitrate Uses
Cadmium nitrate finds application in different industries, including:
1. Electroplating: Cadmium nitrate creates a corrosion-resistant layer on various metal surfaces.
2. Cadmium Pigments: It is used to manufacture cadmium-based pigments for dyes and paints.
3. Cadmium Sulfide Nanoparticles: Nanotechnology synthesises cadmium sulfide nanoparticles with diverse applications in electronics and solar cells.
4. Catalysis: Cadmium nitrate is employed as a catalyst in some chemical reactions, particularly in synthesising organic compounds.
5. Laboratory Reagent: It is used as a reagent in chemical laboratories for various experimental purposes.
| Related Links | |
| Calcium Bromide Formula | Carbon Monoxide Formula |
| Calcium Iodide Formula | Chloroacetic Acid Formula |
Cadmium Nitrate Formula FAQs
How do you write cadmium nitrate?
What is the formula for cadmium nitride?
What is the correct name for Cd(NO3)2?
What is the name of CdBr2?










