CBSE Class 11 Chemistry Notes Chapter 7: The term "chemical equilibrium" describes the point at which neither the reactants nor the products may alter anymore. The advancing and backward reaction rates stay the same in this scenario. It becomes crucial for students to understand equilibrium to understand more complex subjects. The equilibrium chapter notes for Chemistry 11 offer a comprehensive explanation of it.
CBSE Class 11 Chemistry Notes Chapter 7 PDF
Throughout their studies, students must pay close attention to all the important details. These kinds of ideas are necessary, especially for the several kinds of chemical equations that students will encounter. Students can always obtain free PDF resources from the official website, such as the class 11-chapter 7 Chemistry notes, if extra clarification is needed on any topic covered in this chapter.CBSE Class 11 Chemistry Notes Chapter 7 PDF
CBSE Class 11 Chemistry Notes Chapter 7
An essential component of chemical and biological processes is chemical equilibrium. When a liquid evaporates in a closed container, some of the vapour phase's liquid molecules impact the liquid surface and stay in the liquid phase, while molecules with comparatively higher kinetic energy leave the liquid surface into the vapour phase. Because of an equilibrium where the number of molecules leaving the liquid equals the number returning to the liquid from the vapour, it results in a constant vapour pressure. At this point, the system has attained a condition of equilibrium. The rate of evaporation and the rate of condensation are so identical at equilibrium. It could be shown as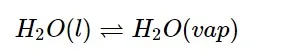 The double arrow above shows that both directions of the procedure are being carried out at the same time. The term "equilibrium mixture" refers to the mixture of reactants and products in the equilibrium state.
The double arrow above shows that both directions of the procedure are being carried out at the same time. The term "equilibrium mixture" refers to the mixture of reactants and products in the equilibrium state.
Equilibrium in the Physical Process
The most important and familiar example is the phase transformation process. Eg.
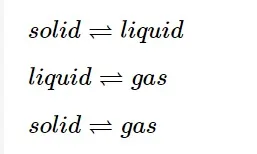
Solid-liquid Equilibrium
The ice and water are in equilibrium at a specific temperature and pressure. The temperature at which the liquid and solid phases of any pure substance are in equilibrium under atmospheric pressure is known as the substance's normal melting or freezing point. When the system reaches dynamic equilibrium, it will behave as follows:- Simultaneously, the opposite processes take place
- The process proceeds at an identical pace, maintaining a steady volume of water and ice.
Liquid-vapour equilibrium
The number of water molecules from the gaseous state into the liquid state increases until the equilibrium is reached. i.e rate of evaporation = rate of condensation The pressure at which water molecules stay constant at a specific temperature is known as the equilibrium water pressure, and it rises as the temperature does. Liquid evaporation is dependent upon,-
The nature of the liquid
-
The amount of liquid
-
The applied temperature
Solid-vapour Equilibrium

General Characteristics of Equilibrium Involving Physical Processes
The system in equilibrium for which the physical processes previously outlined have the following qualities in common:- Only closed systems can achieve equilibrium at a given temperature.
- In steady conditions, the system is dynamic if the rates of the opposing processes are equal.
- Every measurable property in a system stays the same.
- In the case of a physical process, equilibrium is defined as the constant value of one of its parameters.
Equilibrium in Chemical Processes
Chemical reactions, in contrast to physical systems, also reach a state of equilibrium. Both forward and reverse motion are possible for such chemical processes. When the rate of forward reaction equals the rate of backward reaction, there is a dynamic chemical equilibrium.Dynamic Nature of Chemical Equilibrium
This dynamic nature of chemical equilibrium can be explained by the synthesis of ammonia by Haber’s process. This process starts with definite amounts of 𝑁2 𝑎𝑛𝑑𝐻2 and carries out a reaction when equilibrium is attained at a particular temperature. At equilibrium the concentrations of N 2 a n d N H 3 𝑁2 𝑎𝑛𝑑𝑁𝐻3 are constant.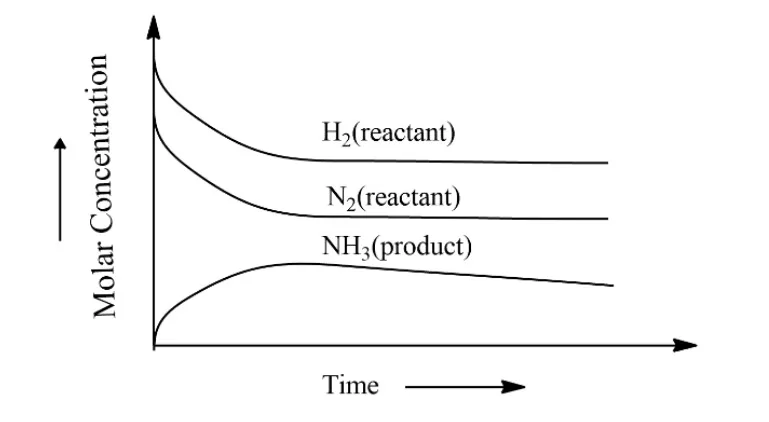
Characteristics of Chemical Equilibrium
At equilibrium, the concentrations of all the reactants and products are constant. At equilibrium, the rates of forward and backward reactions are equal, indicating that equilibrium is dynamic. Only chemical equilibrium can be established if none of the products are permitted to escape or separate as solids.Equilibrium Constant:
An equilibrium mixture is a mixture of reactants and products in the equilibrium state.
Consider a general reversible reaction,

Characteristics of Equilibrium Constant
The value of the equilibrium constant for a given reaction is always constant; it is not affected by the starting reactant concentrations or the direction in which the equilibrium approached. Instead, it solely depends on the reaction's temperature. When the reaction is reversed, the equilibrium constant's value is inverted.Le Chatelier’s Principle
This principle aids in choosing the appropriate course of action and in qualitatively predicting how conditions will change to affect equilibrium. It says that "a system will change in such a manner as to reduce or counteract the effect of the change if any of the factors that determine the equilibrium conditions of a system change." Any physical or chemical equilibrium system can benefit from this idea.Benefits of CBSE Class 11 Chemistry Notes Chapter 7
Since equilibrium indicates the direction of chemical processes, it is crucial to our understanding of chemical reactions. We can manipulate the reaction circumstances to promote the creation of the desired products with the aid of the equilibrium. Calculating the final reaction mixture's composition can also benefit from it. Hence these notes will provide you with every detail about the equilibrium and how can you solve these questions easily.CBSE Class 11 Chemistry Notes Chapter 7 FAQs
What is Chapter 7 in chemistry Class 11?
What is the unit 7 of equilibrium?
What is the best short note of equilibrium?